Surfaces and Interfaces ( IF 5.7 ) Pub Date : 2023-12-09 , DOI: 10.1016/j.surfin.2023.103740
Siyan Wang , Jing Wang , Zhiqiang Wang , Litao Zhang , Hongyan Xu
|
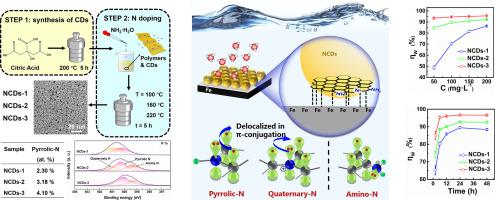
In this study, nitrogen-doped carbon dots (NCDs) were synthesized using a two-step hydrothermal method. Initially, carbon dots (CDs) were prepared from citric acid, followed by nitrogen doping with ammonia solution. By varying the heating temperature in the second step, three types of NCDs were synthesized containing amino-N, pyrrolic-N, or quaternary-N. The corrosion inhibition properties of these NCDs on Q235 carbon steel in 1 M HCl were investigated. The NCDs with the highest pyrrolic-N content (4.10 at.%) demonstrated superior corrosion inhibition, with an efficiency of 96.63 % at 200 mg/L, underscoring the vital role of pyrrolic-N. Quantum chemical calculations elucidated the underlying mechanism: NCDs adsorbed parallelly onto the carbon steel surface by donating their π-electrons to Fe atoms, and forming a protective film that segregated the steel from the acid solution. The delocalization of p-orbital electrons from pyrrolic-N into π-conjugation altered the energies of the π-electrons within the NCDs, enhancing their reactivity and electron-donation ability, thereby augmenting their adsorption capability.
中文翻译:

吡咯氮对氮掺杂碳点缓蚀性能的影响
在这项研究中,采用两步水热法合成了氮掺杂碳点(NCD)。最初,用柠檬酸制备碳点(CD),然后用氨溶液掺杂氮。通过改变第二步中的加热温度,合成了含有氨基-N、吡咯-N或季-N的三种类型的NCD。研究了这些 NCD 在 1 M HCl 中对 Q235 碳钢的缓蚀性能。吡咯氮含量最高 (4.10 at.%) 的 NCD 表现出优异的缓蚀作用,在 200 mg/L 浓度下的效率为 96.63%,凸显了吡咯氮的重要作用。量子化学计算阐明了潜在的机制:NCD 通过将 π 电子提供给 Fe 原子,平行吸附到碳钢表面,并形成一层保护膜,将钢与酸溶液隔离。 p轨道电子从吡咯-N离域到π-共轭改变了NCD内π-电子的能量,增强了它们的反应性和电子供给能力,从而增强了它们的吸附能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号