Phytomedicine ( IF 6.7 ) Pub Date : 2023-12-08 , DOI: 10.1016/j.phymed.2023.155267 Rümeysa Yücer , Shaimaa Fayez , Doris Feineis , Sabine M. Klauck , Letian Shan , Gerhard Bringmann , Thomas Efferth , Mona Dawood
|
Background
Inhibition of NF-κB activity represents a strategy to treat acute myeloid leukemia, one of the most lethal leukemia types. Naphthylisoquinolines (NIQs) are cytotoxic alkaloids from lianas of the families Ancistrocladaceae and Dioncophyllaceae, which are indigenous to tropical rainforests.
Purpose
Uncovering therapeutic possibilities and underlying molecular mechanisms of dioncophylline A and its derivatives towards NF-κB related cellular processes.
Methods
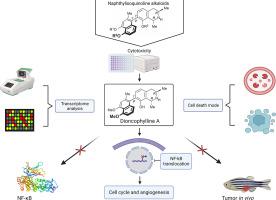
Resazurin-based cell viability assay was performed for dioncophylline A and three derivatives on wild-type CCRF-CEM and multidrug-resistant CEM/ADR5000 cells. Transcriptome analysis was executed to discover cellular functions and molecular networks associated with dioncophylline A treatment. Expression changes obtained by mRNA microarray hybridization were confirmed using qRT-PCR. Molecular docking was applied to predict the affinity of the NIQs with NF-κB. To validate the in silico approach, NF-κB reporter assays were conducted on HEK-BlueTM Null1 cells. Cell death mechanisms and cell cycle arrest were studied using flow cytometry. The potential activity on angiogenesis was evaluated with the endothelial cell tube formation assay on HUVECs using fluorescence microscopy. Intracellular NF-κB location in HEK-BlueTM Null1 cells was visualized with immunofluorescence. Finally, the anti-tumor activity of dioncophylline A was studied by a xenograft zebrafish model in vivo.
Results
Our study demonstrated that dioncophylline A and its derivatives exerted potent cytotoxicity on leukemia cells. Using Ingenuity Pathway Analysis, we identified the NF-κB network as the top network, and docking experiments predicted dioncophylline A and two of its derivatives sharing the same binding pocket with the positive control compound, triptolide. Dioncophylline A showed the best inhibitory activity in NF-κB reporter assays compared to its derivatives, caused autophagy rather than apoptosis, and induced G2/M arrest. It also prevented NF-κB translocation from the cytoplasm to the nucleus. Tube formation as an angiogenesis marker was significantly suppressed by dioncophylline A treatment. Finally, the remarkable anti-tumor activity of dioncophylline A was proven in zebrafish in vivo.
Conclusion
Taken together, we report for the first time the molecular mechanism behind the cytotoxic effect of dioncophylline A on leukemia cells. Dioncophylline A showed strong cytotoxic activity, inhibited NF-κB translocation, significantly affected the NF-κB in silico and in vitro, subdued tube formation, induced autophagy, and exerted antitumor activity in vivo. Our findings enlighten both the cellular functions including the NF-κB signaling pathway and the cytotoxic mechanism affected by dioncophylline A.
中文翻译:

通过 NF-κB 抑制、血管生成抑制、G2/M 细胞周期阻滞和自噬诱导介导的二酚茶碱 A 和相关萘基异喹啉对白血病细胞的细胞毒性
背景
抑制 NF-κB 活性是治疗急性髓性白血病(最致命的白血病类型之一)的一种策略。萘基异喹啉 (NIQ) 是来自 Ancistrocladaceae 和 Dioncophyllaceae 科藤本植物的细胞毒性生物碱,这些藤本植物原产于热带雨林。
目的
揭示二酚茶碱 A 及其衍生物对 NF-κB 相关细胞过程的治疗可能性和潜在分子机制。
方法
在野生型 CCRF-CEM 和多重耐药 CEM/ADR5000 细胞上对二酚茶碱 A 和三种衍生物进行了基于刃天青的细胞活力测定。进行转录组分析以发现与二酚茶碱 A 治疗相关的细胞功能和分子网络。使用 qRT-PCR 确认通过 mRNA 微阵列杂交获得的表达变化。应用分子对接来预测 NIQ 与 NF-κB 的亲和力。为了验证计算机方法,对 HEK-Blue TM Null1 细胞进行了 NF-κB 报告基因分析。使用流式细胞术研究细胞死亡机制和细胞周期停滞。使用荧光显微镜对 HUVEC 进行内皮细胞管形成测定,评估其对血管生成的潜在活性。通过免疫荧光可视化HEK-Blue TM Null1 细胞中的细胞内 NF-κB 位置。最后,通过斑马鱼异种移植模型在体内研究了二酚茶碱A的抗肿瘤活性。
结果
我们的研究表明,二酚茶碱 A 及其衍生物对白血病细胞具有有效的细胞毒性。利用 Ingenuity Pathway Analysis,我们确定 NF-κB 网络为顶层网络,对接实验预测二酚茶碱 A 及其两种衍生物与阳性对照化合物雷公藤甲素具有相同的结合袋。与其衍生物相比,二酚茶碱 A 在 NF-κB 报告基因检测中表现出最佳的抑制活性,引起自噬而不是细胞凋亡,并诱导 G2/M 期阻滞。它还阻止 NF-κB 从细胞质易位到细胞核。苯茶碱 A 治疗显着抑制了作为血管生成标志物的管形成。最后,在斑马鱼体内证实了二酚茶碱A显着的抗肿瘤活性。
结论
综上所述,我们首次报道了二酚茶碱 A 对白血病细胞的细胞毒作用背后的分子机制。Dioncophylline A 显示出强大的细胞毒活性,抑制 NF-κB 易位,在计算机和体外显着影响 NF-κB ,抑制管形成,诱导自噬,并在体内发挥抗肿瘤活性。我们的研究结果揭示了包括 NF-κB 信号通路在内的细胞功能和受二酚茶碱 A 影响的细胞毒性机制。








































 京公网安备 11010802027423号
京公网安备 11010802027423号