Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
干酪根热解微观机理的ReaxFF分子动力学研究
Langmuir ( IF 3.7 ) Pub Date : 2023-12-07 , DOI: 10.1021/acs.langmuir.3c02908
Yongzhong Chen 1 , Zhentao Wang 1, 2 , Bin Li 1 , Kai Yu 1 , Hai Wang 1 , Jue Wang 1 , Yuanping Huo 1 , Junfeng Wang 1
Affiliation
|
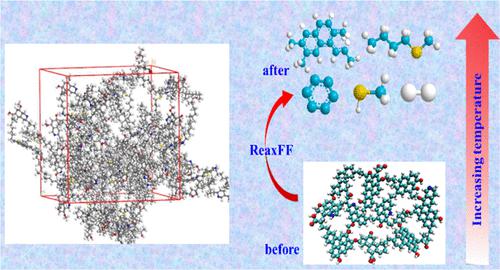
干酪根主要由碳、氢、氧组成,是原油和天然气的主要成分。干酪根热解是产生清洁能源的有效方法。本工作利用ReaxFF分子动力学(MD)方法模拟了三种干酪根的热解反应过程,研究其微观机理和产物分布。结果表明,三类干酪根的热解产物显着依赖于分子结构、温度和反应时间。随着温度升高,气态烃以及轻油和重油馏分减少,其中小分子碎片聚合形成新的分子碎片。在等温条件下,随着反应的进行,热解碎片中的某些组分发生聚合,从而产生新的轻油和重油。此外,还利用量子化学分析揭示了干酪根热解机理。首先,C-O、C-N、C-S结构等弱键被分解,生成大的碳和一些重质页岩油碎片。其次,环烷烃和长链烷烃分解,生成大量轻质页岩油和气态烃。最后发生芳环中C=C的分解,发生轻质和重质页岩油的二次分解,以及短链烷烃的进一步分解。此外,氢气(H 2 )的产生发生在热解反应的后期。 C-H键分解形成氢自由基,随后相互碰撞,形成H 2分子。 干酪根的热解和化学分析可以明确确定烃类物质的类型和含量,为页岩气和页岩油的勘探、开发和利用提供科学数据。

"点击查看英文标题和摘要"































 京公网安备 11010802027423号
京公网安备 11010802027423号