当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Nirmatrelvir: Development of a Scalable Cobalt-Catalyzed Cyclopropanation for Manufacture of the Bicyclic [3.1.0]Proline-Building Block
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-12-04 , DOI: 10.1021/acs.oprd.3c00251 Russell F. Algera 1 , Christophe Allais 1 , Aaron F. Baldwin 2 , Torsten Busch 3 , Federica Colombo 4 , Matteo Colombo 4 , Christelle Depretz 5 , Yves René Dumond 5 , Andres R. Faria Quintero 1 , Maria Heredia 2 , Jörg Jung 3 , Amrita Lall 2 , Taegyo Lee 1 , Yizhou Liu 2 , Stefano Mandelli 4 , Marvin Mantel 3 , Ronald Morris 2 , Jason Mustakis 1 , Bao Nguyen 2 , Robert Pearson 1 , Jared L. Piper 1 , John A. Ragan 1 , Brigitte Ruffin 5 , Courtney Talicska 2 , Sergei Tcyrulnikov 1 , Christopher Uyeda 6 , R. Matthew Weekly 1 , Ming Zeng 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2023-12-04 , DOI: 10.1021/acs.oprd.3c00251 Russell F. Algera 1 , Christophe Allais 1 , Aaron F. Baldwin 2 , Torsten Busch 3 , Federica Colombo 4 , Matteo Colombo 4 , Christelle Depretz 5 , Yves René Dumond 5 , Andres R. Faria Quintero 1 , Maria Heredia 2 , Jörg Jung 3 , Amrita Lall 2 , Taegyo Lee 1 , Yizhou Liu 2 , Stefano Mandelli 4 , Marvin Mantel 3 , Ronald Morris 2 , Jason Mustakis 1 , Bao Nguyen 2 , Robert Pearson 1 , Jared L. Piper 1 , John A. Ragan 1 , Brigitte Ruffin 5 , Courtney Talicska 2 , Sergei Tcyrulnikov 1 , Christopher Uyeda 6 , R. Matthew Weekly 1 , Ming Zeng 2
Affiliation
|
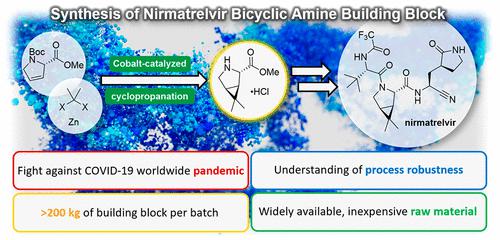
Nirmatrelvir is a potent, selective, and orally bioavailable inhibitor of SARS-CoV-2 Mpro. Herein, we report a scalable cyclopropanation to produce the bicyclic [3.1.0]proline derivative, which is one of the key starting materials for the synthesis of nirmatrelvir. To ensure a robust supply chain for this building block and meet the significant API demand, we needed to develop a synthetic process that was complementary to existing strategies. To achieve this goal, we used widely available and inexpensive raw material trans-(2S,4R)-4-hydroxy-l-proline as a starting material and implemented a recently reported cobalt-catalyzed gem-dimethylcyclopropanation at the manufacturing scale. Mechanistic studies led to the identification of potential catalyst decomposition pathways and provided insights into the key reaction parameters. This process was demonstrated to produce >200 kg of the bicyclic [3.1.0]proline derivative per batch and multi-metric ton quantities overall.
中文翻译:

Nirmatrelvir 的合成:开发可扩展的钴催化环丙烷化反应,用于制造双环 [3.1.0] 脯氨酸结构单元
Nirmatrelvir 是一种有效的、选择性的、口服生物可利用的 SARS-CoV-2 M pro 抑制剂。在此,我们报道了一种可扩展的环丙烷化反应来生产双环[3.1.0]脯氨酸衍生物,这是合成尼马瑞韦的关键起始材料之一。为了确保该构建模块拥有强大的供应链并满足重要的 API 需求,我们需要开发一种与现有策略相补充的合成工艺。为了实现这一目标,我们使用广泛可用且廉价的原材料反式-(2S,4R)-4-羟基-脯氨酸作为起始材料,并在制造规模上实施了最近报道的钴催化的偕二甲基环丙烷化。机理研究确定了潜在的催化剂分解途径,并提供了对关键反应参数的见解。经证明,该工艺每批次可生产 >200 kg 的双环[3.1.0]脯氨酸衍生物,总体产量可达多吨。
更新日期:2023-12-04
中文翻译:

Nirmatrelvir 的合成:开发可扩展的钴催化环丙烷化反应,用于制造双环 [3.1.0] 脯氨酸结构单元
Nirmatrelvir 是一种有效的、选择性的、口服生物可利用的 SARS-CoV-2 M pro 抑制剂。在此,我们报道了一种可扩展的环丙烷化反应来生产双环[3.1.0]脯氨酸衍生物,这是合成尼马瑞韦的关键起始材料之一。为了确保该构建模块拥有强大的供应链并满足重要的 API 需求,我们需要开发一种与现有策略相补充的合成工艺。为了实现这一目标,我们使用广泛可用且廉价的原材料反式-(2S,4R)-4-羟基-脯氨酸作为起始材料,并在制造规模上实施了最近报道的钴催化的偕二甲基环丙烷化。机理研究确定了潜在的催化剂分解途径,并提供了对关键反应参数的见解。经证明,该工艺每批次可生产 >200 kg 的双环[3.1.0]脯氨酸衍生物,总体产量可达多吨。































 京公网安备 11010802027423号
京公网安备 11010802027423号