当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inorganic Polysulfide Chemistries for Better Energy Storage Systems
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-12-07 , DOI: 10.1021/acs.accounts.3c00484 Xiaona Li 1 , Xueliang Sun 1 , Biwei Xiao 2, 3 , Deping Wang 4 , Jianwen Liang 2, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2023-12-07 , DOI: 10.1021/acs.accounts.3c00484 Xiaona Li 1 , Xueliang Sun 1 , Biwei Xiao 2, 3 , Deping Wang 4 , Jianwen Liang 2, 3
Affiliation
|
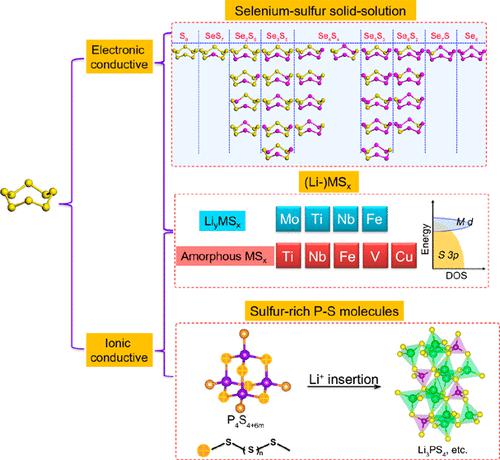
Sulfur-based cathode materials have become a research hot spot as one of the most promising candidates for next-generation, high-energy lithium batteries. However, the insulating nature of elemental sulfur or organosulfides has become the biggest challenge that leads to dramatic degradation and hinders their practical application. The disadvantage is more obvious for all-solid-state battery systems, which require both high electronic and ionic migration at the same sites to realize a complete electrochemical reaction. In addition to adding conductive components into the cathode composites, another effective way to realize high-reversibility sulfur-based cathodes is by optimizing the inherent nature of sulfur-based materials to make them “conductive”. Inorganic polysulfide materials including polysulfide molecules, selenium-sulfur solid solutions, and (lithium) metal polysulfides are promising, as they have different structures that can make them intrinsically conductive or becoming conductive during lithiation. They all contain at least one −S–S– bridged bond, which is the intrinsic structural characteristic and the source of the chemical properties of these polysulfide compounds. For example, by balancing the conductivity and reversible capacity, researchers in the US National Aeronautics and Space Administration (NASA) have shown that 500 Wh/kg solid-state Li–Se/S batteries can power cars and even electric aircraft.
中文翻译:

无机多硫化物化学物质可实现更好的储能系统
硫基正极材料作为下一代高能锂电池最有希望的候选材料之一,已成为研究热点。然而,元素硫或有机硫化物的绝缘性质已成为导致其急剧降解并阻碍其实际应用的最大挑战。对于全固态电池系统来说,缺点更为明显,因为全固态电池系统需要在同一位点同时进行高电子和离子迁移才能实现完整的电化学反应。除了在正极复合材料中添加导电成分之外,实现高可逆性硫基正极的另一个有效途径是优化硫基材料的固有性质,使其具有“导电性”。包括多硫化物分子、硒硫固溶体和(锂)金属多硫化物在内的无机多硫化物材料很有前景,因为它们具有不同的结构,可以使其本身导电或在锂化过程中变得导电。它们都含有至少一个-S-S-桥键,这是这些多硫化合物的内在结构特征和化学性质的来源。例如,通过平衡电导率和可逆容量,美国国家航空航天局(NASA)的研究人员表明,500 Wh/kg的固态Li-Se/S电池可以为汽车甚至电动飞机提供动力。
更新日期:2023-12-07
中文翻译:

无机多硫化物化学物质可实现更好的储能系统
硫基正极材料作为下一代高能锂电池最有希望的候选材料之一,已成为研究热点。然而,元素硫或有机硫化物的绝缘性质已成为导致其急剧降解并阻碍其实际应用的最大挑战。对于全固态电池系统来说,缺点更为明显,因为全固态电池系统需要在同一位点同时进行高电子和离子迁移才能实现完整的电化学反应。除了在正极复合材料中添加导电成分之外,实现高可逆性硫基正极的另一个有效途径是优化硫基材料的固有性质,使其具有“导电性”。包括多硫化物分子、硒硫固溶体和(锂)金属多硫化物在内的无机多硫化物材料很有前景,因为它们具有不同的结构,可以使其本身导电或在锂化过程中变得导电。它们都含有至少一个-S-S-桥键,这是这些多硫化合物的内在结构特征和化学性质的来源。例如,通过平衡电导率和可逆容量,美国国家航空航天局(NASA)的研究人员表明,500 Wh/kg的固态Li-Se/S电池可以为汽车甚至电动飞机提供动力。































 京公网安备 11010802027423号
京公网安备 11010802027423号