当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkali Metal Cation–Sulfate Anion Ion Pairs Promoted the Cleavage of C–C Bond During Ethanol Electrooxidation
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-12-06 , DOI: 10.1021/acs.jpclett.3c02569 Xiang Li 1 , Guangxing Yang 1 , Qiao Zhang 1 , Zhiting Liu 1 , Feng Peng 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-12-06 , DOI: 10.1021/acs.jpclett.3c02569 Xiang Li 1 , Guangxing Yang 1 , Qiao Zhang 1 , Zhiting Liu 1 , Feng Peng 1
Affiliation
|
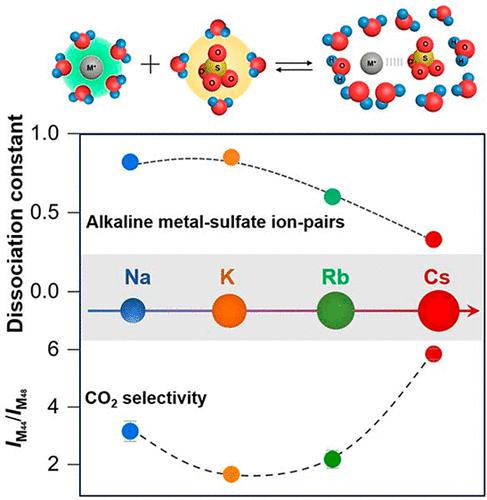
Direct ethanol fuel cells show great promise as a means of converting biomass ethanol derived from biomass into electricity. However, the efficiency of complete conversion is hindered by the low selectivity in breaking the C–C bond. This selectivity is determined by factors such as the material structure and reaction conditions, including the nature of the supporting electrolyte. Cations serve not only as facilitators of electricity conduction through ion migration but also as influencers of the reaction pathways. In this study, we utilized differential electrochemical mass spectrometry to track the in situ generation of CO2 during potential scanning. The presence of alkali cations led to an enhancement in the CO2 selectivity. In addition, in situ Raman spectroscopy provided evidence of the formation of alkali metal cation–sulfate anion ion pairs. The catalytic activity and CO2 selectivity were found to be directly correlated to the ionic strength of these ion pairs.
中文翻译:

乙醇电氧化过程中碱金属阳离子-硫酸根阴离子离子对促进C-C键断裂
直接乙醇燃料电池作为将源自生物质的生物质乙醇转化为电力的一种手段显示出巨大的前景。然而,C-C键断裂选择性低,阻碍了完全转化的效率。这种选择性由材料结构和反应条件等因素决定,包括支持电解质的性质。阳离子不仅通过离子迁移充当电传导的促进剂,而且还充当反应途径的影响者。在本研究中,我们利用差示电化学质谱法来追踪电位扫描过程中CO 2的原位生成。碱金属阳离子的存在导致CO 2选择性的增强。此外,原位拉曼光谱提供了碱金属阳离子-硫酸根阴离子对形成的证据。发现催化活性和CO 2选择性与这些离子对的离子强度直接相关。
更新日期:2023-12-06
中文翻译:

乙醇电氧化过程中碱金属阳离子-硫酸根阴离子离子对促进C-C键断裂
直接乙醇燃料电池作为将源自生物质的生物质乙醇转化为电力的一种手段显示出巨大的前景。然而,C-C键断裂选择性低,阻碍了完全转化的效率。这种选择性由材料结构和反应条件等因素决定,包括支持电解质的性质。阳离子不仅通过离子迁移充当电传导的促进剂,而且还充当反应途径的影响者。在本研究中,我们利用差示电化学质谱法来追踪电位扫描过程中CO 2的原位生成。碱金属阳离子的存在导致CO 2选择性的增强。此外,原位拉曼光谱提供了碱金属阳离子-硫酸根阴离子对形成的证据。发现催化活性和CO 2选择性与这些离子对的离子强度直接相关。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号