当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective P450BM3 Hydroxylation of Cyclobutylamine and Bicyclo[1.1.1]pentylamine Derivatives: Underpinning Synthetic Chemistry for Drug Discovery
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-05 , DOI: 10.1021/jacs.3c10542 Lucy A Harwood 1 , Ziyue Xiong 2 , Kirsten E Christensen 1 , Ruiyao Wang 3 , Luet L Wong 2, 4 , Jeremy Robertson 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-05 , DOI: 10.1021/jacs.3c10542 Lucy A Harwood 1 , Ziyue Xiong 2 , Kirsten E Christensen 1 , Ruiyao Wang 3 , Luet L Wong 2, 4 , Jeremy Robertson 1, 2
Affiliation
|
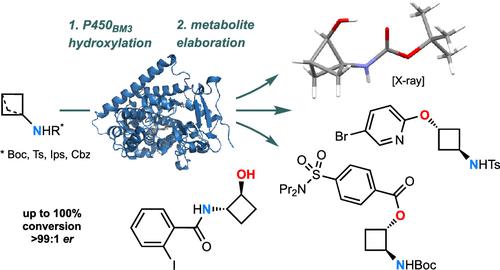
Achieving single-step syntheses of a set of related compounds divergently and selectively from a common starting material affords substantial efficiency gains when compared with preparing those same compounds by multiple individual syntheses. In order for this approach to be realized, complementary reagent systems must be available; here, a panel of engineered P450BM3 enzymes is shown to fulfill this remit in the selective C–H hydroxylation of cyclobutylamine derivatives at chemically unactivated sites. The oxidations can proceed with high regioselectivity and stereoselectivity, producing valuable bifunctional intermediates for synthesis and applications in fragment-based drug discovery. The process also applies to bicyclo[1.1.1]pentyl (BCP) amine derivatives to achieve the first direct enantioselective functionalization of the bridging methylenes and open a short and efficient route to chiral BCP bioisosteres for medicinal chemistry. The combination of substrate, enzyme, and reaction engineering provides a powerful general platform for small-molecule elaboration and diversification.
中文翻译:

环丁胺和双环[1.1.1]戊胺衍生物的选择性 P450BM3 羟基化:支撑药物发现的合成化学
与通过多次单独合成制备那些相同的化合物相比,从共同的起始材料中不同地、选择性地实现一组相关化合物的单步合成可提供显着的效率增益。为了实现这种方法,必须提供补充试剂系统;在这里,一组工程化的 P450 BM3酶被证明可以在化学未激活位点对环丁胺衍生物进行选择性 C-H 羟基化,从而实现这一任务。氧化可以以高区域选择性和立体选择性进行,产生有价值的双功能中间体,用于基于片段的药物发现的合成和应用。该工艺还适用于双环[1.1.1]戊基(BCP)胺衍生物,首次实现桥连亚甲基的直接对映选择性官能化,并为药物化学手性BCP生物等排体开辟了一条短而有效的途径。底物、酶和反应工程的结合为小分子精加工和多样化提供了强大的通用平台。
更新日期:2023-12-05
中文翻译:

环丁胺和双环[1.1.1]戊胺衍生物的选择性 P450BM3 羟基化:支撑药物发现的合成化学
与通过多次单独合成制备那些相同的化合物相比,从共同的起始材料中不同地、选择性地实现一组相关化合物的单步合成可提供显着的效率增益。为了实现这种方法,必须提供补充试剂系统;在这里,一组工程化的 P450 BM3酶被证明可以在化学未激活位点对环丁胺衍生物进行选择性 C-H 羟基化,从而实现这一任务。氧化可以以高区域选择性和立体选择性进行,产生有价值的双功能中间体,用于基于片段的药物发现的合成和应用。该工艺还适用于双环[1.1.1]戊基(BCP)胺衍生物,首次实现桥连亚甲基的直接对映选择性官能化,并为药物化学手性BCP生物等排体开辟了一条短而有效的途径。底物、酶和反应工程的结合为小分子精加工和多样化提供了强大的通用平台。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号