Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-12-05 , DOI: 10.1016/j.bmc.2023.117543 Juan Zhang 1 , Zhen Wang 1 , Conghao Gai 2 , Fan Yang 3 , Xiaoqing Yun 1 , Boye Jiang 1 , Yan Zou 2 , Qingguo Meng 3 , Qingjie Zhao 2 , Xiaoyun Chai 2
|
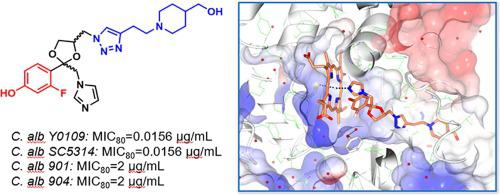
In order to develop antifungal drugs, a series of novel azole analogues were designed and synthesized based on our previous work. Most of the target compounds had broad-spectrum antifungal activity, which showed excellent to moderate inhibitory activity against the tested strains, except A. fum 0504656. Among these, compounds B3, B7, B8, B11, B12 and E9 showed excellent activity against C. alb Y0109 and C. alb SC5314 (with the MIC80: 0.0156 ug/mL). In addition, compound B3 showed the best inhibitory activity against fluconazole-resistant strains C. alb 901 and C. alb 904, and had low toxicity against NIH/3T3 cells at the effective MIC range against fungi. Structure-activity relationship and docking studies of the derivatives suggest that the presence of the 2-fluoro-4-hydroxyphenyl and 1,2,3-triazole group enhance the antifungal activity of the compounds, which may be related to the interaction of the key groups with the amino acids surrounding the target enzyme.
中文翻译:

作为有效抗真菌剂的新型唑类似物的设计、合成、评估和优化
为了开发抗真菌药物,我们在前期工作的基础上设计合成了一系列新型唑类类似物。大多数目标化合物具有广谱抗真菌活性,对除A. fum 0504656 之外的测试菌株均表现出优异至中等的抑制活性。其中,化合物B3 、 B7 、 B8 、 B11 、 B12和E9对C. fum 表现出优异的活性。 . alb Y0109 和C. alb SC5314(MIC 80 :0.0156 ug/mL)。此外,化合物B3对氟康唑耐药菌株C. alb 901和C. alb 904表现出最佳的抑制活性,并且在对真菌的有效MIC范围内对NIH/3T3细胞具有较低的毒性。衍生物的构效关系和对接研究表明,2-氟-4-羟基苯基和1,2,3-三唑基团的存在增强了化合物的抗真菌活性,这可能与关键化合物的相互作用有关。与目标酶周围的氨基酸组成的基团。































 京公网安备 11010802027423号
京公网安备 11010802027423号