当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vesicle-to-micelle transition in a double chain quaternary ammonium surfactant system: Interfacial behavior and molecular insights
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-12-06 , DOI: 10.1016/j.molliq.2023.123714 Jiani Gong , Yao Song , Yujia Sun , Qihan Sun , Changyao Liu , Jiajing Tan , Li Zhao , Baocai Xu
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-12-06 , DOI: 10.1016/j.molliq.2023.123714 Jiani Gong , Yao Song , Yujia Sun , Qihan Sun , Changyao Liu , Jiajing Tan , Li Zhao , Baocai Xu
|
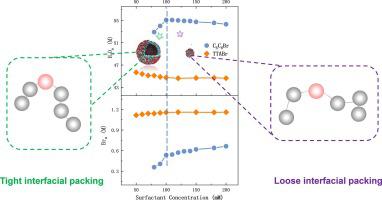
Vesicles have emerged as promising drug carriers with excellent compatibility with cell membranes, holding immense potential for biomedical applications. While most reported micelle-to-vesicle transitions involve increasing surfactant or additive concentrations, the reverse vesicle-to-micelle transition remains less explored. In this study, we investigate the vesicle-to-micelle transition of hexyldimethyloctylammonium bromide (CCBr) using dynamic light scattering, transmission electron microscopy, and fluorescence spectroscopy. Tetradecyltrimethylammonium bromide (TTABr), a single-chain tetraalkylammonium surfactant with the same chain-length, is studied for comparison. Through the chemical trapping (CT) method, we explore the interfacial composition changes during the vesicle-to-micelle transition. Notably, CCBr exhibits a unique characteristic setting it apart from TTABr and other cationic surfactants. At lower concentration ranges, there is a simultaneous increase in interfacial molarities of water and bromide ions, signifying a decrease in interfacial headgroup molarity and surfactant molecule packing. Moreover, our dissipative particle dynamics (DPD) simulation results show that, as the surfactant concentration rises, the interior angle between surfactant chains widens, and the molecular configuration of CCBr approaches a straight line. These combined CT and DPD findings underscore the prominent role of steric effects arising from the rigidity of the double-chain. These effects become more pronounced at higher CCBr concentrations, leading to looser interfacial packing of surfactant monomers and driving the transition from vesicles to micelles. Interestingly, the breakage of vesicles was not observed when replacing the counterion of CCBr with acetate in CCAc solutions. CT results suggest that the elevated interfacial acetate molarity may faciliate the compact packing of surfactant monomers and hinder vesicle-to-micelle transformation. Our study provides a molecular-level understanding of the vesicle-to-micelle transitions in CCBr solutions and establishes a theoretical basis for morphological control of surfactant aggregates.
中文翻译:

双链季铵表面活性剂体系中的囊泡到胶束的转变:界面行为和分子见解
囊泡已成为一种有前景的药物载体,与细胞膜具有良好的相容性,在生物医学应用方面具有巨大的潜力。虽然大多数报道的胶束到囊泡的转变涉及增加表面活性剂或添加剂浓度,但反向囊泡到胶束的转变仍然很少被探索。在这项研究中,我们利用动态光散射、透射电子显微镜和荧光光谱研究了己基二甲基辛基溴化铵 (CCBr) 的囊泡到胶束的转变。为了进行比较,研究了具有相同链长的单链四烷基铵表面活性剂十四烷基三甲基溴化铵(TTABr)。通过化学捕获(CT)方法,我们探索了囊泡到胶束转变过程中界面组成的变化。值得注意的是,CCBr 表现出独特的特性,使其有别于 TTABr 和其他阳离子表面活性剂。在较低浓度范围内,水和溴离子的界面摩尔浓度同时增加,这表明界面头基摩尔浓度和表面活性剂分子堆积降低。此外,我们的耗散粒子动力学(DPD)模拟结果表明,随着表面活性剂浓度的升高,表面活性剂链之间的内角变宽,CCBr的分子构型接近直线。这些 CT 和 DPD 的综合结果强调了双链刚性产生的空间效应的显着作用。这些效应在 CCBr 浓度较高时变得更加明显,导致表面活性剂单体的界面堆积更加松散,并推动从囊泡向胶束的转变。有趣的是,当在 CCAc 溶液中用乙酸盐替换 CCBr 的抗衡离子时,没有观察到囊泡的破裂。 CT结果表明,界面乙酸摩尔浓度升高可能有利于表面活性剂单体的紧密堆积,并阻碍囊泡向胶束的转化。我们的研究提供了对 CCBr 溶液中囊泡到胶束转变的分子水平理解,并为表面活性剂聚集体的形态控制奠定了理论基础。
更新日期:2023-12-06
中文翻译:

双链季铵表面活性剂体系中的囊泡到胶束的转变:界面行为和分子见解
囊泡已成为一种有前景的药物载体,与细胞膜具有良好的相容性,在生物医学应用方面具有巨大的潜力。虽然大多数报道的胶束到囊泡的转变涉及增加表面活性剂或添加剂浓度,但反向囊泡到胶束的转变仍然很少被探索。在这项研究中,我们利用动态光散射、透射电子显微镜和荧光光谱研究了己基二甲基辛基溴化铵 (CCBr) 的囊泡到胶束的转变。为了进行比较,研究了具有相同链长的单链四烷基铵表面活性剂十四烷基三甲基溴化铵(TTABr)。通过化学捕获(CT)方法,我们探索了囊泡到胶束转变过程中界面组成的变化。值得注意的是,CCBr 表现出独特的特性,使其有别于 TTABr 和其他阳离子表面活性剂。在较低浓度范围内,水和溴离子的界面摩尔浓度同时增加,这表明界面头基摩尔浓度和表面活性剂分子堆积降低。此外,我们的耗散粒子动力学(DPD)模拟结果表明,随着表面活性剂浓度的升高,表面活性剂链之间的内角变宽,CCBr的分子构型接近直线。这些 CT 和 DPD 的综合结果强调了双链刚性产生的空间效应的显着作用。这些效应在 CCBr 浓度较高时变得更加明显,导致表面活性剂单体的界面堆积更加松散,并推动从囊泡向胶束的转变。有趣的是,当在 CCAc 溶液中用乙酸盐替换 CCBr 的抗衡离子时,没有观察到囊泡的破裂。 CT结果表明,界面乙酸摩尔浓度升高可能有利于表面活性剂单体的紧密堆积,并阻碍囊泡向胶束的转化。我们的研究提供了对 CCBr 溶液中囊泡到胶束转变的分子水平理解,并为表面活性剂聚集体的形态控制奠定了理论基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号