当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanochemical Iron-Catalyzed Nitrene Transfer Reactions: Direct Synthesis of N-Acyl Sulfonimidamides from Sulfinamides and Dioxazolones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-12-06 , DOI: 10.1002/anie.202316702 Shulei Pan 1 , Florian F Mulks 1 , Peng Wu 1 , Kari Rissanen 2 , Carsten Bolm 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-12-06 , DOI: 10.1002/anie.202316702 Shulei Pan 1 , Florian F Mulks 1 , Peng Wu 1 , Kari Rissanen 2 , Carsten Bolm 1
Affiliation
|
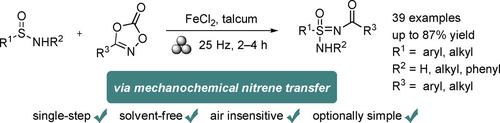
A mechanochemical nitrene transfer reaction towards N-acyl sulfonimidamides from sulfinamides and dioxazolones with the catalysis of iron(II) chloride is presented. This one-step solvent-free procedure shows better conversions rate and chemoselectivity compared to its solution-phase counterpart, providing a wide range of N-acyl sulfonimidamides in up to 87 % yield. Mechanistically, crucial nitrene iron complex intermediates are suggested by ESI-MS.
中文翻译:

机械化学铁催化氮烯转移反应:由亚磺酰胺和二恶唑酮直接合成 N-酰基磺酰亚胺
提出了在氯化铁 (II) 催化下,从亚磺酰胺和二恶唑酮向 N-酰基磺酰亚胺进行机械化学氮宾转移反应。与溶液相方法相比,这一一步式无溶剂程序显示出更好的转化率和化学选择性,可提供多种N-酰基磺酰亚胺,收率高达 87%。从机理上讲,ESI-MS 提示了关键的氮宾铁络合物中间体。
更新日期:2023-12-06
中文翻译:

机械化学铁催化氮烯转移反应:由亚磺酰胺和二恶唑酮直接合成 N-酰基磺酰亚胺
提出了在氯化铁 (II) 催化下,从亚磺酰胺和二恶唑酮向 N-酰基磺酰亚胺进行机械化学氮宾转移反应。与溶液相方法相比,这一一步式无溶剂程序显示出更好的转化率和化学选择性,可提供多种N-酰基磺酰亚胺,收率高达 87%。从机理上讲,ESI-MS 提示了关键的氮宾铁络合物中间体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号