当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Mechanism of Installation of 5-Carboxymethylaminomethyl Uridine Hypermodification by tRNA-Modifying Enzymes MnmE and MnmG
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-05 , DOI: 10.1021/jacs.3c10182
Praneeth Bommisetti 1 , Vahe Bandarian 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-12-05 , DOI: 10.1021/jacs.3c10182
Praneeth Bommisetti 1 , Vahe Bandarian 1
Affiliation
|
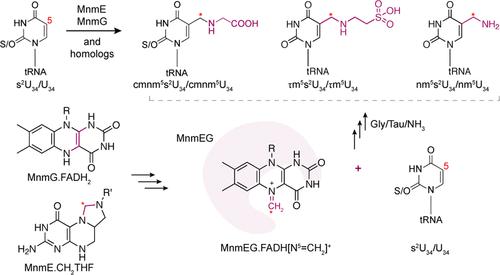
The evolutionarily conserved bacterial proteins MnmE and MnmG (and their homologues in Eukarya) install a 5-carboxymethylaminomethyl (cmnm5) or a 5-taurinomethyl (τm5) group onto wobble uridines of several tRNA species. The Escherichia coli MnmE binds guanosine-5′-triphosphate (GTP) and methylenetetrahydrofolate (CH2THF), while MnmG binds flavin adenine dinucleotide (FAD) and a reduced nicotinamide adenine dinucleotide (NADH). Together with glycine, MnmEG catalyzes the installation of cmnm5 in a reaction that also requires hydrolysis of GTP. In this letter, we investigated key steps of the MnmEG reaction using a combination of biochemical techniques. We show multiple lines of evidence supporting flavin-iminium FADH[N5═CH2]+ as a central intermediate in the MnmEG reaction. Using a synthetic FADH[N5═CD2]+ analogue, the intermediacy of the FAD in the transfer of the methylene group from CH2THF to the C5 position of U34 was unambiguously demonstrated. Further, MnmEG reactions containing the deuterated flavin-iminium intermediate and alternate nucleophiles such as taurine and ammonia also led to the formation of the anticipated U34-modified tRNAs, showing FAD[N5═CH2]+ as the universal intermediate for all MnmEG homologues. Additionally, an RNA-protein complex stable to urea-denaturing polyacrylamide gel electrophoresis was identified. Studies involving a series of nuclease (RNase T1) and protease (trypsin) digestions along with reverse transcription experiments suggest that the complex may be noncovalent. While the conserved MnmG cysteine C47 and C277 mutant variants were shown to reduce FAD, they were unable to promote the modified tRNA formation. Overall, this study provides critical insights into the biochemical mechanism underlying tRNA modification by the MnmEG.
中文翻译:

深入了解 tRNA 修饰酶 MnmE 和 MnmG 安装 5-羧甲基氨基甲基尿苷过修饰的机制
进化上保守的细菌蛋白 MnmE 和 MnmG(及其在真核生物中的同源物)将 5-羧甲基氨基甲基 (cmnm5) 或 5-牛磺酰甲基 (τm5) 基团安装到几种 tRNA 种类的摆动尿苷上。大肠杆菌 MnmE 结合鸟苷-5′-三磷酸 (GTP) 和亚甲基四氢叶酸 (CH2THF),而 MnmG 结合黄素腺嘌呤二核苷酸 (FAD) 和还原的烟酰胺腺嘌呤二核苷酸 (NADH)。MnmEG 与甘氨酸一起催化 cmnm5 在也需要 GTP 水解的反应中形成。在这封信中,我们使用生化技术的组合研究了 MnmEG 反应的关键步骤。我们展示了多条证据线支持黄素-亚胺 FADH[N5═CH2]+ 作为 MnmEG 反应中的中心中间体。使用合成的 FADH[N5═CD2]+ 类似物,明确证明了 FAD 在亚甲基从 CH2THF 转移到 U34 的 C5 位置的中介作用。此外,含有氘代黄素-亚胺中间体和替代亲核试剂(如牛磺酸和氨)的 MnmEG 反应也导致形成预期的 U34 修饰的 tRNA,显示 FAD[N5═CH2]+ 是所有 MnmEG 同源物的通用中间体。此外,还鉴定出一种对尿素变性聚丙烯酰胺凝胶电泳稳定的 RNA-蛋白质复合物。涉及一系列核酸酶 (RNase T1) 和蛋白酶 (胰蛋白酶) 消化以及逆转录实验的研究表明,该复合物可能是非共价的。 虽然保守的 MnmG 半胱氨酸 C47 和 C277 突变变体被证明可以减少 FAD,但它们无法促进修饰的 tRNA 形成。总体而言,本研究为 MnmEG 修饰 tRNA 的生化机制提供了重要见解。
更新日期:2023-12-05
中文翻译:

深入了解 tRNA 修饰酶 MnmE 和 MnmG 安装 5-羧甲基氨基甲基尿苷过修饰的机制
进化上保守的细菌蛋白 MnmE 和 MnmG(及其在真核生物中的同源物)将 5-羧甲基氨基甲基 (cmnm5) 或 5-牛磺酰甲基 (τm5) 基团安装到几种 tRNA 种类的摆动尿苷上。大肠杆菌 MnmE 结合鸟苷-5′-三磷酸 (GTP) 和亚甲基四氢叶酸 (CH2THF),而 MnmG 结合黄素腺嘌呤二核苷酸 (FAD) 和还原的烟酰胺腺嘌呤二核苷酸 (NADH)。MnmEG 与甘氨酸一起催化 cmnm5 在也需要 GTP 水解的反应中形成。在这封信中,我们使用生化技术的组合研究了 MnmEG 反应的关键步骤。我们展示了多条证据线支持黄素-亚胺 FADH[N5═CH2]+ 作为 MnmEG 反应中的中心中间体。使用合成的 FADH[N5═CD2]+ 类似物,明确证明了 FAD 在亚甲基从 CH2THF 转移到 U34 的 C5 位置的中介作用。此外,含有氘代黄素-亚胺中间体和替代亲核试剂(如牛磺酸和氨)的 MnmEG 反应也导致形成预期的 U34 修饰的 tRNA,显示 FAD[N5═CH2]+ 是所有 MnmEG 同源物的通用中间体。此外,还鉴定出一种对尿素变性聚丙烯酰胺凝胶电泳稳定的 RNA-蛋白质复合物。涉及一系列核酸酶 (RNase T1) 和蛋白酶 (胰蛋白酶) 消化以及逆转录实验的研究表明,该复合物可能是非共价的。 虽然保守的 MnmG 半胱氨酸 C47 和 C277 突变变体被证明可以减少 FAD,但它们无法促进修饰的 tRNA 形成。总体而言,本研究为 MnmEG 修饰 tRNA 的生化机制提供了重要见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号