当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formal Total Synthesis of Batrachotoxin Enabled by Radical and Weix Coupling Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-05 , DOI: 10.1021/acs.joc.3c02290 Yuuki Watanabe 1 , Komei Sakata 1 , Daisuke Urabe 1 , Koichi Hagiwara 1 , Masayuki Inoue 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-12-05 , DOI: 10.1021/acs.joc.3c02290 Yuuki Watanabe 1 , Komei Sakata 1 , Daisuke Urabe 1 , Koichi Hagiwara 1 , Masayuki Inoue 1
Affiliation
|
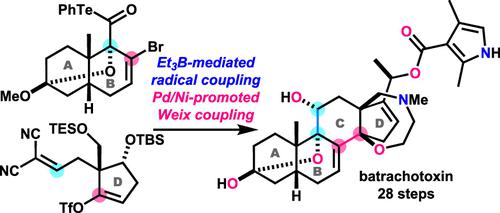
Batrachotoxin (1), originally isolated from a Columbian poison-dart frog, is a steroidal alkaloid. Its 6/6/6/5-membered carbocycle (ABCD-ring) contains two double bonds, one nitrogen, and five oxygen functionalities. We developed a radical-based convergent strategy and realized the total synthesis of 1 in 28 steps. The AB-ring and D-ring fragments were efficiently synthesized and linked by exploiting a powerful Et3B/O2-mediated radical coupling reaction. Vinyl triflate and vinyl bromide were then utilized for a Pd/Ni-promoted Weix coupling reaction to cyclize the C-ring. A hydroxy group of the C-ring was stereoselectively installed by a decarboxylative hydroxylation reaction to prepare an advanced intermediate of our previous total synthesis of 1.
中文翻译:

通过自由基和Weix偶联反应正式全合成箭毒毒素
蝙蝠毒素 ( 1 ) 最初是从哥伦比亚箭毒蛙中分离出来的,是一种类固醇生物碱。其 6/6/6/5 元碳环(ABCD 环)包含两个双键、一个氮和五个氧官能团。我们开发了一种基于激进的收敛策略,并通过28步实现了1的全合成。通过利用强大的Et 3 B/O 2介导的自由基偶联反应,有效合成并连接AB环和D环片段。然后利用三氟甲磺酸乙烯酯和溴乙烯进行 Pd/Ni 促进的 Weix 偶联反应以环化 C 环。通过脱羧羟基化反应立体选择性地安装C环上的羟基,制备了我们之前全合成1的高级中间体。
更新日期:2023-12-05
中文翻译:

通过自由基和Weix偶联反应正式全合成箭毒毒素
蝙蝠毒素 ( 1 ) 最初是从哥伦比亚箭毒蛙中分离出来的,是一种类固醇生物碱。其 6/6/6/5 元碳环(ABCD 环)包含两个双键、一个氮和五个氧官能团。我们开发了一种基于激进的收敛策略,并通过28步实现了1的全合成。通过利用强大的Et 3 B/O 2介导的自由基偶联反应,有效合成并连接AB环和D环片段。然后利用三氟甲磺酸乙烯酯和溴乙烯进行 Pd/Ni 促进的 Weix 偶联反应以环化 C 环。通过脱羧羟基化反应立体选择性地安装C环上的羟基,制备了我们之前全合成1的高级中间体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号