当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Structure and Dynamics Near the Surfaces of Silicalite-1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-12-04 , DOI: 10.1021/acs.jpcc.3c05723 Ankur Agarwal 1 , Jeffrey D. Rimer 1 , Jeremy C. Palmer 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-12-04 , DOI: 10.1021/acs.jpcc.3c05723 Ankur Agarwal 1 , Jeffrey D. Rimer 1 , Jeremy C. Palmer 1
Affiliation
|
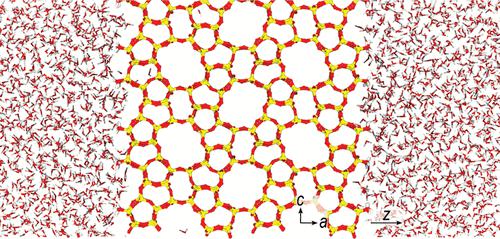
Zeolites are crystalline microporous aluminosilicates that are commonly used as industrial sorbents and membranes. Solvent structuring and dynamics near zeolite crystal surfaces are thought to influence interfacial transport and molecular recognition processes, as well as fundamental aspects of their crystallization from solution. Here, we use molecular dynamics (MD) simulations to investigate the behavior of interfacial water near the exposed (010), (100), and (101) crystal faces of silicalite-1, one of the most widely studied zeolites. The MD simulations reveal that water’s translational and orientational order is strongly influenced by the distinct corrugations, pore apertures, and functional group distributions presented on each surface. Specifically, we observe two distinct hydration layers near each surface. Water molecules in the contact layer are hydrogen bonded to two or three exposed surface silanol groups. The relative populations of water molecules that are doubly and triply hydrogen bonded to the surface strongly depend on the distribution of the exposed silanols. The interactions with exposed surface silanols also influence water’s local orientational order and distribution across each surface. We also show that the structuring of the solvent near the different faces of silicalite-1 strongly impacts the interfacial dynamics. The translational and orientational relaxation dynamics of water are slowest in the contact layers and correlate with structural ordering near each face.
中文翻译:

Silicalite-1 表面附近的水结构和动力学
沸石是结晶微孔铝硅酸盐,通常用作工业吸附剂和膜。沸石晶体表面附近的溶剂结构和动力学被认为影响界面传输和分子识别过程,以及它们从溶液中结晶的基本方面。在这里,我们使用分子动力学 (MD) 模拟来研究 Silalite-1(研究最广泛的沸石之一)暴露的 (010)、(100) 和 (101) 晶面附近界面水的行为。 MD 模拟表明,水的平移和取向顺序受到每个表面上不同波纹、孔径和官能团分布的强烈影响。具体来说,我们观察到每个表面附近有两个不同的水化层。接触层中的水分子与两个或三个暴露的表面硅烷醇基团形成氢键。与表面形成双重和三重氢键的水分子的相对数量很大程度上取决于暴露的硅烷醇的分布。与暴露的表面硅烷醇的相互作用也会影响水的局部取向顺序和在每个表面上的分布。我们还表明,silicalite-1 不同面附近的溶剂结构强烈影响界面动力学。水的平移和定向弛豫动力学在接触层中最慢,并且与每个面附近的结构排序相关。
更新日期:2023-12-04
中文翻译:

Silicalite-1 表面附近的水结构和动力学
沸石是结晶微孔铝硅酸盐,通常用作工业吸附剂和膜。沸石晶体表面附近的溶剂结构和动力学被认为影响界面传输和分子识别过程,以及它们从溶液中结晶的基本方面。在这里,我们使用分子动力学 (MD) 模拟来研究 Silalite-1(研究最广泛的沸石之一)暴露的 (010)、(100) 和 (101) 晶面附近界面水的行为。 MD 模拟表明,水的平移和取向顺序受到每个表面上不同波纹、孔径和官能团分布的强烈影响。具体来说,我们观察到每个表面附近有两个不同的水化层。接触层中的水分子与两个或三个暴露的表面硅烷醇基团形成氢键。与表面形成双重和三重氢键的水分子的相对数量很大程度上取决于暴露的硅烷醇的分布。与暴露的表面硅烷醇的相互作用也会影响水的局部取向顺序和在每个表面上的分布。我们还表明,silicalite-1 不同面附近的溶剂结构强烈影响界面动力学。水的平移和定向弛豫动力学在接触层中最慢,并且与每个面附近的结构排序相关。































 京公网安备 11010802027423号
京公网安备 11010802027423号