当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Acute and Repeated Dose Toxicity Profiling of Chelidonic Acid in Rats: in Silico and in Vivo Evidence
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-11-20 , DOI: 10.1002/cbdv.202301241 Shraddha I Khairnar 1 , Yogesh A Kulkarni 1 , S Murugesan 2 , Kavita Singh 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-11-20 , DOI: 10.1002/cbdv.202301241 Shraddha I Khairnar 1 , Yogesh A Kulkarni 1 , S Murugesan 2 , Kavita Singh 1
Affiliation
|
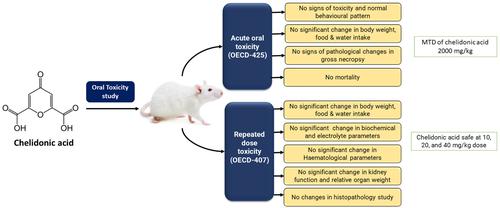
Chelidonic acid is a phytoconstituent found in rhizomes of the perennial plant celandine. The current study aims to evaluate the acute and repeated dose oral toxicity study of chelidonic acid as per the OECD guidelines 425 and 407. The pharmacokinetic and toxicity profile of chelidonic acid was predicted using online servers and tools. A single dose of chelidonic acid (2000 mg/kg) was administered to female Wistar rats in an acute toxicity study, and the animals were monitored for 14 days. We studied the toxicity profile of chelidonic acid at 10, 20, and 40 mg/kg doses in Wistar rats for repeated dose toxicity (28 days). Clinical biochemistry, haematological, and urine parameters were estimated. A gross necropsy and histopathology were performed. A single oral dose of chelidonic acid (2000 mg/kg) showed no signs of toxicity or mortality. The Administration of chelidonic acid showed no significant alterations in haematological, biochemical, and urine parameters. The histopathology showed normal structure and architecture in all the vital organs. A gross necropsy of vital organs showed no signs of toxicity. The chelidonic acid was found to be safe at all selected dose levels in the acute and repeated dose toxicity study in rats.
中文翻译:

白屈菜酸对大鼠的急性和重复剂量毒性分析的影响:计算机和体内证据
白屈菜酸是一种在多年生植物白屈菜的根茎中发现的植物成分。本研究旨在根据 OECD 指南 425 和 407 评估白屈菜酸的急性和重复剂量口服毒性研究。使用在线服务器和工具预测白屈菜酸的药代动力学和毒性特征。在一项急性毒性研究中,对雌性 Wistar 大鼠施用单剂量白屈菜酸 (2000 mg/kg),并对动物进行为期 14 天的监测。我们研究了 Wistar 大鼠中 10、20 和 40 mg/kg 剂量的白屈菜酸的重复剂量毒性(28 天)的毒性特征。评估临床生化、血液学和尿液参数。进行了大体尸检和组织病理学检查。单次口服剂量的白屈菜酸(2000 mg/kg)没有显示出毒性或死亡迹象。白屈菜酸的施用未显示血液学、生化和尿液参数的显着改变。组织病理学显示所有重要器官的结构和结构正常。重要器官的大体尸检显示没有毒性迹象。在大鼠急性和重复剂量毒性研究中发现白屈菜酸在所有选定剂量水平下都是安全的。
更新日期:2023-11-20
中文翻译:

白屈菜酸对大鼠的急性和重复剂量毒性分析的影响:计算机和体内证据
白屈菜酸是一种在多年生植物白屈菜的根茎中发现的植物成分。本研究旨在根据 OECD 指南 425 和 407 评估白屈菜酸的急性和重复剂量口服毒性研究。使用在线服务器和工具预测白屈菜酸的药代动力学和毒性特征。在一项急性毒性研究中,对雌性 Wistar 大鼠施用单剂量白屈菜酸 (2000 mg/kg),并对动物进行为期 14 天的监测。我们研究了 Wistar 大鼠中 10、20 和 40 mg/kg 剂量的白屈菜酸的重复剂量毒性(28 天)的毒性特征。评估临床生化、血液学和尿液参数。进行了大体尸检和组织病理学检查。单次口服剂量的白屈菜酸(2000 mg/kg)没有显示出毒性或死亡迹象。白屈菜酸的施用未显示血液学、生化和尿液参数的显着改变。组织病理学显示所有重要器官的结构和结构正常。重要器官的大体尸检显示没有毒性迹象。在大鼠急性和重复剂量毒性研究中发现白屈菜酸在所有选定剂量水平下都是安全的。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号