当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic CO2 Reduction Coupled with Oxidation of Benzyl Alcohol over CsPbBr3@PANI Nanocomposites
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-12-04 , DOI: 10.1021/acs.jpclett.3c02766
Fuwei Chen 1 , Ziquan Li 1 , Yueming Jiang 1 , Zhen Li 1 , Ruosheng Zeng 2 , Ziyi Zhong 3, 4 , Ming-De Li 1 , Jin Z Zhang 5 , Binbin Luo 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-12-04 , DOI: 10.1021/acs.jpclett.3c02766
Fuwei Chen 1 , Ziquan Li 1 , Yueming Jiang 1 , Zhen Li 1 , Ruosheng Zeng 2 , Ziyi Zhong 3, 4 , Ming-De Li 1 , Jin Z Zhang 5 , Binbin Luo 1
Affiliation
|
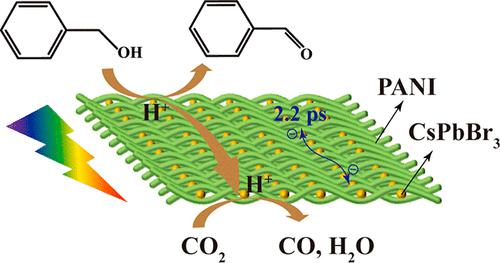
Herein, we successfully prepare conductive polyaniline (PANI)-encapsulated CsPbBr3 perovskite nanocrystals (PNCs) that demonstrate much improved photocatalytic performance and stability toward the CO2 reduction reaction (CRR) coupled with oxidation of benzyl alcohol (BA) to benzaldehyde. Due to the acid–base interaction between CO2 and PANI, CO2 molecules are selectively adsorbed on PANI in the form of carbamate. As a result, the rate of production of CO (rCO) reaches 26.1 μmol g–1 h–1 with a selectivity of 98.1%, which is in good agreement with the rate of oxidation (∼27.0 μmol g–1 h–1) of BA. Such a high reduction/oxidation rate is enabled by the fast electron transfer (∼2.2 ps) from PNCs to PANI, as revealed by femtosecond transient absorption spectroscopy. Moreover, because of the benefit of the encapsulation of PANI, no significant decrease in rCO is observed in a 10 h CRR test. This work offers insight into how to simultaneously achieve improved photocatalytic performance and stability of CsPbX3 PNCs.
中文翻译:

CsPbBr3@PANI 纳米复合材料上光催化 CO2 还原与苯甲醇氧化耦合
在此,我们成功制备了导电聚苯胺(PANI)封装的CsPbBr 3钙钛矿纳米晶体(PNC),该纳米晶体表现出大大改善的光催化性能和对CO 2还原反应(CRR)以及苯甲醇(BA)氧化成苯甲醛的稳定性。由于CO 2和PANI之间的酸碱相互作用,CO 2分子以氨基甲酸酯的形式选择性吸附在PANI上。结果,CO ( r CO ) 的生成速率达到 26.1 μmol g –1 h –1 ,选择性为 98.1%,这与氧化速率 (∼27.0 μmol g –1 h –1 )的学士学位。飞秒瞬态吸收光谱显示,如此高的还原/氧化速率是通过从 PNC 到 PANI 的快速电子转移(~2.2 ps)实现的。此外,由于 PANI 封装的好处,在 10 小时 CRR 测试中没有观察到rCO显着下降。这项工作深入了解了如何同时提高 CsPbX 3 PNC 的光催化性能和稳定性。
更新日期:2023-12-04
中文翻译:

CsPbBr3@PANI 纳米复合材料上光催化 CO2 还原与苯甲醇氧化耦合
在此,我们成功制备了导电聚苯胺(PANI)封装的CsPbBr 3钙钛矿纳米晶体(PNC),该纳米晶体表现出大大改善的光催化性能和对CO 2还原反应(CRR)以及苯甲醇(BA)氧化成苯甲醛的稳定性。由于CO 2和PANI之间的酸碱相互作用,CO 2分子以氨基甲酸酯的形式选择性吸附在PANI上。结果,CO ( r CO ) 的生成速率达到 26.1 μmol g –1 h –1 ,选择性为 98.1%,这与氧化速率 (∼27.0 μmol g –1 h –1 )的学士学位。飞秒瞬态吸收光谱显示,如此高的还原/氧化速率是通过从 PNC 到 PANI 的快速电子转移(~2.2 ps)实现的。此外,由于 PANI 封装的好处,在 10 小时 CRR 测试中没有观察到rCO显着下降。这项工作深入了解了如何同时提高 CsPbX 3 PNC 的光催化性能和稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号