当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pyrene-bridged acenaphthenes: synthesis and properties of a diacenaphtho[1,2-e:1′,2′-l]pyrene and its symmetrical nitrogen analogue
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-12-04 , DOI: 10.1039/d3ob01744c Jonas Polkaehn 1 , Peter Ehlers 1 , Alexander Villinger 1 , Peter Langer 1, 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2023-12-04 , DOI: 10.1039/d3ob01744c Jonas Polkaehn 1 , Peter Ehlers 1 , Alexander Villinger 1 , Peter Langer 1, 2
Affiliation
|
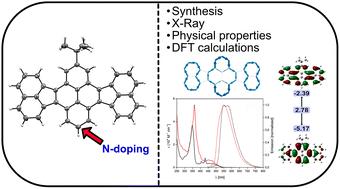
The considerable need for novel polyaromatic hydrocarbons (PAHs) for applications in the area of organic electronics remains unchanged. Diacenaphthopyrene represents a new PAH consisting of two acenaphthylene units connected by a pyrene bridge. The system is built up by Pd-catalyzed cross-coupling, followed by acid catalyzed cyclosiomerization to generate the pyrene moiety. The new fused scaffold is formed in the last step in convincing yields by means of CH-activation. We additionally synthesized one aza-pyrene based analogue. The two hitherto unknown PAHs were investigated in detail by UV-Vis and PL spectroscopy, CV measurements and DFT calculations. Based on these results, the abilities of the novel structure as well as the effect of incorporation of nitrogen were evaluated.
中文翻译:

芘桥苊:二苊并[1,2-e:1′,2′-l]芘及其对称氮类似物的合成和性质
有机电子领域对新型多环芳烃(PAH)的巨大需求仍然没有改变。二苊并芘是一种新的多环芳烃,由两个苊单元通过芘桥连接而成。该系统是通过 Pd 催化的交叉偶联建立的,然后通过酸催化的环化反应生成芘部分。新的融合支架是在最后一步通过 CH 激活形成的,其产量令人信服。我们还合成了一种基于氮杂芘的类似物。通过 UV-Vis 和 PL 光谱、CV 测量和 DFT 计算对两种迄今为止未知的 PAH 进行了详细研究。基于这些结果,评估了新结构的能力以及氮掺入的效果。
更新日期:2023-12-04
中文翻译:

芘桥苊:二苊并[1,2-e:1′,2′-l]芘及其对称氮类似物的合成和性质
有机电子领域对新型多环芳烃(PAH)的巨大需求仍然没有改变。二苊并芘是一种新的多环芳烃,由两个苊单元通过芘桥连接而成。该系统是通过 Pd 催化的交叉偶联建立的,然后通过酸催化的环化反应生成芘部分。新的融合支架是在最后一步通过 CH 激活形成的,其产量令人信服。我们还合成了一种基于氮杂芘的类似物。通过 UV-Vis 和 PL 光谱、CV 测量和 DFT 计算对两种迄今为止未知的 PAH 进行了详细研究。基于这些结果,评估了新结构的能力以及氮掺入的效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号