当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cooperation of ferrous ions and hydrated ferric oxide for advanced phosphate removal over a wide pH range: Mechanism and kinetics
Water Research ( IF 11.4 ) Pub Date : 2023-12-03 , DOI: 10.1016/j.watres.2023.120969 Xiaohui Wang 1 , Yang Li 1 , Xue Wen 1 , Liyan Liu 2 , Lizhi Zhang 1 , Mingce Long 1
Water Research ( IF 11.4 ) Pub Date : 2023-12-03 , DOI: 10.1016/j.watres.2023.120969 Xiaohui Wang 1 , Yang Li 1 , Xue Wen 1 , Liyan Liu 2 , Lizhi Zhang 1 , Mingce Long 1
Affiliation
|
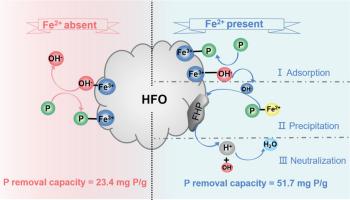
Excessive phosphate loading leads to eutrophication problems in rivers or lakes and causes serious environmental and economic damages, urging new technologies to reduce effluent phosphate at ultra-low levels. As a promising candidate, adsorption over metal oxides is restricted by the released hydroxide anions (OH− ) through ligand exchange, which elevates pH and suppresses further adsorption. In this contribution, we found ferrous ions (Fe2+ ) significantly enhance phosphate removal over hydrated ferric oxide (HFO) in a wide pH range via a cooperation of adsorption and precipitation, and clarified the synergistic mechanism by a series of characterizations and the modified models of adsorption isotherms and pseudo second-order kinetics. The combination of Fe2+ and HFO removed up to 51.7 mg/g of phosphate at pH 4.0, with 43.6 and 8.1 mg/g attributing to adsorption and precipitation, respectively. In comparison to HFO alone, HFO/Fe2+ system achieved 2.2-fold increase in phosphate removal, 1.9-fold increase in phosphate adsorption capacity, and 3.4-fold increase in phosphate removal rate. The enhancement is understood by that hydroxide anions released from ligand exchange over HFO are neutralized by protons produced from the oxidative precipitation of ferrous ions. The HFO/Fe2+ combining system is promising to realize advanced removal of low concentration phosphate containing wastewater, and these findings bring new insights for the development of novel phosphate removal technologies through a rational design of a combination process.
中文翻译:

亚铁离子和水合三氧化二铁的协同作用,可在较宽的 pH 范围内进行高级磷酸盐去除:机理和动力学
过量的磷酸盐负荷导致河流或湖泊出现富营养化问题,并造成严重的环境和经济损失,迫切需要新技术将出水磷酸盐降低到超低水平。作为一种有前途的候选者,金属氧化物上的吸附受到配体交换释放的氢氧根阴离子(OH−)的限制,这会提高 pH 值并抑制进一步吸附。在这篇文章中,我们发现亚铁离子(Fe2+)通过吸附和沉淀的协同作用,在较宽的pH范围内显着增强水合氧化铁(HFO)的除磷效果,并通过一系列表征和修改模型阐明了协同机制。吸附等温线和伪二级动力学。 Fe2+ 和 HFO 的组合在 pH 4.0 时去除了高达 51.7 mg/g 的磷酸盐,其中吸附和沉淀分别去除了 43.6 和 8.1 mg/g。与单独使用HFO相比,HFO/Fe2+系统的磷酸盐去除率提高了2.2倍,磷酸盐吸附能力提高了1.9倍,磷酸盐去除率提高了3.4倍。这种增强是通过 HFO 上的配体交换释放的氢氧根阴离子被亚铁离子氧化沉淀产生的质子中和来理解的。 HFO/Fe2+组合系统有望实现低浓度含磷废水的深度去除,这些发现为通过组合工艺的合理设计开发新型除磷技术带来了新的见解。
更新日期:2023-12-03
中文翻译:

亚铁离子和水合三氧化二铁的协同作用,可在较宽的 pH 范围内进行高级磷酸盐去除:机理和动力学
过量的磷酸盐负荷导致河流或湖泊出现富营养化问题,并造成严重的环境和经济损失,迫切需要新技术将出水磷酸盐降低到超低水平。作为一种有前途的候选者,金属氧化物上的吸附受到配体交换释放的氢氧根阴离子(OH−)的限制,这会提高 pH 值并抑制进一步吸附。在这篇文章中,我们发现亚铁离子(Fe2+)通过吸附和沉淀的协同作用,在较宽的pH范围内显着增强水合氧化铁(HFO)的除磷效果,并通过一系列表征和修改模型阐明了协同机制。吸附等温线和伪二级动力学。 Fe2+ 和 HFO 的组合在 pH 4.0 时去除了高达 51.7 mg/g 的磷酸盐,其中吸附和沉淀分别去除了 43.6 和 8.1 mg/g。与单独使用HFO相比,HFO/Fe2+系统的磷酸盐去除率提高了2.2倍,磷酸盐吸附能力提高了1.9倍,磷酸盐去除率提高了3.4倍。这种增强是通过 HFO 上的配体交换释放的氢氧根阴离子被亚铁离子氧化沉淀产生的质子中和来理解的。 HFO/Fe2+组合系统有望实现低浓度含磷废水的深度去除,这些发现为通过组合工艺的合理设计开发新型除磷技术带来了新的见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号