当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Synthesis of 3-(Indol-2-yl)quinoxalin-2-ones and 4-(Benzimidazol-2-yl)-3-methyl(aryl)cinnolines via Polyphosphoric Acid (PPA)-Mediated Intramolecular Rearrangements of 3-(Methyl/aryl(2-phenylhydrazono)methyl)quinoxalin-2-ones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-11-30 , DOI: 10.1021/acs.joc.3c01626 Vakhid A Mamedov 1 , Liliya V Mustakimova 1 , Zheng-Wang Qu 2 , Hui Zhu 2 , Victor V Syakaev 1 , Venera R Galimullina 1 , Leisan R Shamsutdinova 1 , Il'dar Kh Rizvanov 1 , Aidar T Gubaidullin 1 , Oleg G Sinyashin 1 , Stefan Grimme 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-11-30 , DOI: 10.1021/acs.joc.3c01626 Vakhid A Mamedov 1 , Liliya V Mustakimova 1 , Zheng-Wang Qu 2 , Hui Zhu 2 , Victor V Syakaev 1 , Venera R Galimullina 1 , Leisan R Shamsutdinova 1 , Il'dar Kh Rizvanov 1 , Aidar T Gubaidullin 1 , Oleg G Sinyashin 1 , Stefan Grimme 2
Affiliation
|
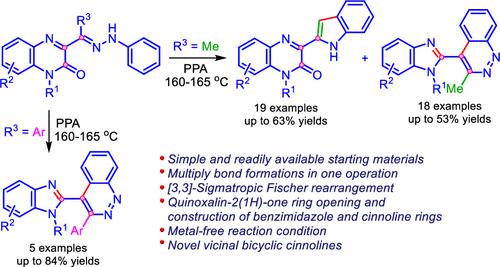
Herein, we report a polyphosphoric acid (PPA)-mediated divergent metal-free operation to access a diverse collection of 3-(indol-2-yl)quinoxalin-2-ones and 4-(benzimidazol-2-yl)-3-methylcinnolines in moderate to excellent overall yields. The described process involves two distinct, and competing rearrangements of 3-(methyl(2-phenylhydrazono)methyl)quinoxalin-2-ones, namely [3,3]-sigmatropic Fischer rearrangement with the formation of an indole ring to produce 3-(indol-2-yl)-quinoxalin-2-ones, and Mamedov rearrangement with simultaneous construction of benzimidazole and cinnoline rings to form the new biheterocyclic system─4-(benzimidazol-2-yl)-3-methylcinnolines. The reaction mechanism of both rearrangement channels is explored by extensive dispersion-corrected DFT calculations. It is partcularly remarkable that when 3-(aryl(2-phenylhydrazono)methyl)quinoxalin-2-ones is used, instead of 3-(methyl(2-phenylhydrazono)methyl)quinoxalin-2-ones, reactions proceed regioselectively with the formation of only rearrangement products─4-(benzimidazol-2-yl)-3-arylcinnolines with high yields. This operationally simple protocol enables a rapid access to these scaffolds and is compatible with a wide scope of starting materials. In addition, the new rearrangement found features a promising approach for the design of unique compound libraries for drug design and discovery programs.
中文翻译:

3-(吲哚-2-基)喹喔啉-2-酮和4-(苯并咪唑-2-基)-3-甲基(芳基)肉啉通过多磷酸(PPA)介导的3-(甲基)分子内重排的发散合成/芳基(2-苯基亚肼基)甲基)喹喔啉-2-酮
在此,我们报告了一种多磷酸(PPA)介导的不同的无金属操作,以获得多种3-(吲哚-2-基)喹喔啉-2-酮和4-(苯并咪唑-2-基)-3-甲基肉啉的总产率中等至优异。所描述的过程涉及 3-(甲基(2-苯基亚肼基)甲基)喹喔啉-2-酮的两个不同且竞争的重排,即 [3,3]-sigmatropic Fischer 重排,形成吲哚环以产生 3-(吲哚-2-基)-喹喔啉-2-酮,以及Mamedov重排同时构建苯并咪唑和肉桂啉环,形成新的双杂环体系─4-(苯并咪唑-2-基)-3-甲基肉啉。通过广泛的色散校正 DFT 计算探索了两个重排通道的反应机制。特别值得注意的是,当使用 3-(芳基(2-苯基亚肼基)甲基)喹喔啉-2-酮代替 3-(甲基(2-苯基亚肼基)甲基)喹喔啉-2-酮时,反应会区域选择性地进行并形成唯一的重排产物─4-(苯并咪唑-2-基)-3-芳基肉啉,产率高。这种操作简单的方案可以快速访问这些支架,并且与多种起始材料兼容。此外,新的重排发现了一种有前途的方法,用于设计用于药物设计和发现项目的独特化合物库。
更新日期:2023-11-30
中文翻译:

3-(吲哚-2-基)喹喔啉-2-酮和4-(苯并咪唑-2-基)-3-甲基(芳基)肉啉通过多磷酸(PPA)介导的3-(甲基)分子内重排的发散合成/芳基(2-苯基亚肼基)甲基)喹喔啉-2-酮
在此,我们报告了一种多磷酸(PPA)介导的不同的无金属操作,以获得多种3-(吲哚-2-基)喹喔啉-2-酮和4-(苯并咪唑-2-基)-3-甲基肉啉的总产率中等至优异。所描述的过程涉及 3-(甲基(2-苯基亚肼基)甲基)喹喔啉-2-酮的两个不同且竞争的重排,即 [3,3]-sigmatropic Fischer 重排,形成吲哚环以产生 3-(吲哚-2-基)-喹喔啉-2-酮,以及Mamedov重排同时构建苯并咪唑和肉桂啉环,形成新的双杂环体系─4-(苯并咪唑-2-基)-3-甲基肉啉。通过广泛的色散校正 DFT 计算探索了两个重排通道的反应机制。特别值得注意的是,当使用 3-(芳基(2-苯基亚肼基)甲基)喹喔啉-2-酮代替 3-(甲基(2-苯基亚肼基)甲基)喹喔啉-2-酮时,反应会区域选择性地进行并形成唯一的重排产物─4-(苯并咪唑-2-基)-3-芳基肉啉,产率高。这种操作简单的方案可以快速访问这些支架,并且与多种起始材料兼容。此外,新的重排发现了一种有前途的方法,用于设计用于药物设计和发现项目的独特化合物库。































 京公网安备 11010802027423号
京公网安备 11010802027423号