Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
聚苯胺对聚苯胺/煤复合材料吸附罗丹明 B 染料协同影响的高级平衡研究
ACS Omega ( IF 3.7 ) Pub Date : 2023-12-01 , DOI: 10.1021/acsomega.3c07355 Mohamed Adel Sayed 1, 2 , Abdelrahman Mohamed 2 , Sayed A Ahmed 2, 3 , Ahmed M El-Sherbeeny 4 , Wail Al Zoubi 5 , Mostafa R Abukhadra 1, 6
Affiliation
|
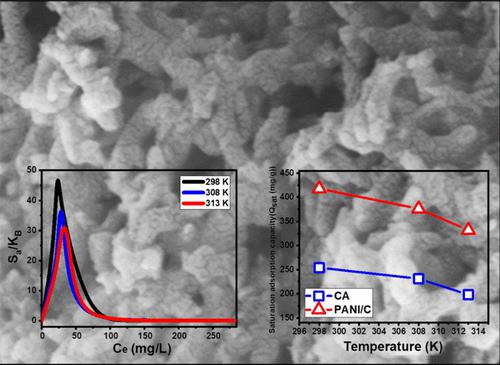
使用传统的平衡模型和先进的等温线研究评估了聚苯胺(PANI)杂化过程对PANI/煤杂化材料(PANI/C)吸附罗丹明B染料(RB)的协同改善效果。该复合材料是通过在煤馏分存在下聚合聚苯胺制备的,表面积为27.7 m 2 /g。与煤颗粒 (254.3 mg/g) 相比,PANI/C 杂化物具有更高的吸附 RB 染料的能力 (423.5 mg/g)。使用活性位点密度 (Nm) 的空间特征以及单个活性位点上吸附的 RB 总数 (n) 说明了 PANI/C 消除性能的持续增加。然而,PANI的加入并没有对现有活性位点的数量产生任何实质性影响,但杂交过程极大地影响了每个活性位点的选择性和亲和力,以及染料与复合材料相互作用时的聚集特性。表面。原煤只能吸附 3 个 RB 分子,而整个 PANI/C 表面的每个活性位点可以吸附大约 8 个 RB 分子。这也是 RB 染料以垂直排列吸附的证据,涉及多分子过程。高斯能(4.01–5.59 kJ/mol)和吸附能(−4.34–4.68 kJ/mol)揭示了物理机制的可控影响。这些机制可能包括范德华力、偶极-偶极相互作用和氢键 (<30 kJ/mol)。已评估的热力学函数(例如焓、内能和熵)提供了支持 PANI/C 吸收 RB 过程的放热和自发性质的证据。

"点击查看英文标题和摘要"


















































 京公网安备 11010802027423号
京公网安备 11010802027423号