当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulating Zn-ion solvation structure and Zn(0 0 2) deposition for stable Zn anode
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-11-29 , DOI: 10.1016/j.cej.2023.147759
Quan Zong , Yifei Yu , Chaofeng Liu , Qiaoling Kang , Bo Lv , Daiwen Tao , Jingji Zhang , Jiangying Wang , Qilong Zhang , Guozhong Cao
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-11-29 , DOI: 10.1016/j.cej.2023.147759
Quan Zong , Yifei Yu , Chaofeng Liu , Qiaoling Kang , Bo Lv , Daiwen Tao , Jingji Zhang , Jiangying Wang , Qilong Zhang , Guozhong Cao
|
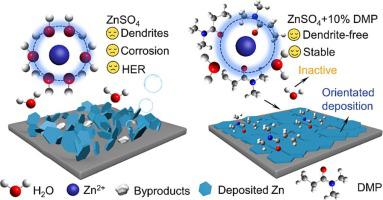
Severe side reactions and Zn dendrite growth are two main causes deteriorating the stability of zinc metal anodes. N,N-dimethylpropionamide (DMP) is investigated as an electrolyte additive to suppress side reactions and regulate Zn deposition. Experimental results and theoretical calculations demonstrate that the DMP additive can facilitate solvation structure of hydrated Zn2+ and electrode–electrolyte interface in aqueous ZnSO4 electrolyte. DMP develops strong coordination with Zn2+ through the carbonyl group to reduce the water activity, consequently alleviating the water-associated side reactions. DMP prefers to adsorb on (14 + 10 % DMP electrolyte enables stable Zn plating/stripping in Zn||Zn symmetric cells with a capacity of 1 mAh cm−2 at 1 mA cm−2 for 4000 h, and offers good reversibility in Zn||Cu cells with an average Coulombic efficiency of 99.7 % over 2000 cycles. The coupled Zn||NH4 V4 O10 full cell using ZnSO4 with 10 % DMP additive exhibits enhanced stability.
中文翻译:

调节 Zn 离子溶剂化结构和 Zn(0 0 2) 沉积以获得稳定的 Zn 阳极
严重的副反应和锌枝晶生长是导致锌金属阳极稳定性恶化的两个主要原因。研究将 N,N-二甲基丙酰胺 (DMP) 作为电解液添加剂来抑制副反应并调节锌沉积。实验结果和理论计算表明,DMP添加剂可以促进水合Zn2+和ZnSO4电解质水溶液中电极-电解质界面的溶剂化结构。 DMP通过羰基与Zn2+形成强配位,降低水活度,从而减轻与水相关的副反应。 DMP更倾向于吸附在(100)和(101)晶面而不是(002)晶面上,从而诱导Zn沿(002)晶面优先生长。结果,ZnSO4 + 10% DMP 电解质能够在容量为 1 mAh cm−2、1 mA cm−2 的 Zn||Zn 对称电池中稳定镀锌/剥离 4000 小时,并在 Zn| 中提供良好的可逆性。 |铜电池在 2000 次循环中平均库仑效率为 99.7%。使用 ZnSO4 和 10% DMP 添加剂的耦合 Zn||NH4V4O10 全电池表现出增强的稳定性。
更新日期:2023-11-29
中文翻译:

调节 Zn 离子溶剂化结构和 Zn(0 0 2) 沉积以获得稳定的 Zn 阳极
严重的副反应和锌枝晶生长是导致锌金属阳极稳定性恶化的两个主要原因。研究将 N,N-二甲基丙酰胺 (DMP) 作为电解液添加剂来抑制副反应并调节锌沉积。实验结果和理论计算表明,DMP添加剂可以促进水合Zn2+和ZnSO4电解质水溶液中电极-电解质界面的溶剂化结构。 DMP通过羰基与Zn2+形成强配位,降低水活度,从而减轻与水相关的副反应。 DMP更倾向于吸附在(100)和(101)晶面而不是(002)晶面上,从而诱导Zn沿(002)晶面优先生长。结果,ZnSO4 + 10% DMP 电解质能够在容量为 1 mAh cm−2、1 mA cm−2 的 Zn||Zn 对称电池中稳定镀锌/剥离 4000 小时,并在 Zn| 中提供良好的可逆性。 |铜电池在 2000 次循环中平均库仑效率为 99.7%。使用 ZnSO4 和 10% DMP 添加剂的耦合 Zn||NH4V4O10 全电池表现出增强的稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号