当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Highly Efficient Novel Antifungal Lead Compounds Targeting Succinate Dehydrogenase: Pyrazole-4-carboxamide Derivatives with an N-Phenyl Substituted Amide Fragment
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-11-29 , DOI: 10.1021/acs.jafc.3c04842 Xin-Peng Sun 1, 2 , Chen-Sheng Yu 1 , Li-Jing Min 3 , Charles L Cantrell 4 , Xuewen Hua 5 , Na-Bo Sun 2 , Xing-Hai Liu 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2023-11-29 , DOI: 10.1021/acs.jafc.3c04842 Xin-Peng Sun 1, 2 , Chen-Sheng Yu 1 , Li-Jing Min 3 , Charles L Cantrell 4 , Xuewen Hua 5 , Na-Bo Sun 2 , Xing-Hai Liu 1
Affiliation
|
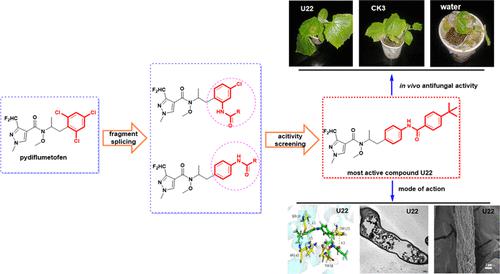
Developing environmentally friendly fungicides is crucial to tackle the issue of rising pesticide resistance. In this study, a series of novel pyrazole-4-carboxamide derivatives containing N-phenyl substituted amide fragments were designed and synthesized. The structures of target compounds were confirmed by 1H NMR, 13C NMR, and HRMS, and the crystal structure of the most active compound N-(1-(4-(4-(tert-butyl)benzamido)phenyl)propan-2-yl)-3-(difluoromethyl)-N-methoxy-1-methyl-1H-pyrazole-4-carboxamide (U22) was further determined by X-ray single-crystal diffraction. The bioassay results indicated that the 26 target compounds possessed good in vitro antifungal activity against Sclerotinia sclerotiorum with EC50 values for compounds U12, U13, U15, U16, U18, U22, and U23 being 4.17 ± 0.46, 8.04 ± 0.71, 7.01 ± 0.71, 12.77 ± 1.00, 8.11 ± 0.70, 0.94 ± 0.11, and 9.48 ± 0.83 μg·mL–1, respectively, which were the similar to controls bixafen (6.70 ± 0.47 μg·mL–1), fluxapyroxad (0.71 ± 0.14 μg·mL–1), and pydiflumetofen (0.06 ± 0.01 μg·mL–1). Furthermore, in vivo antifungal activity results against S. sclerotiorum indicated that compounds U12 (80.6%) and U22 (89.9%) possessed excellent preventative efficacy at 200 μg·mL–1, which was the same as the control pydiflumetofen (82.4%). Scanning electron microscopy and transmission electron microscopy studies found that the compound U22 could destroy the hyphal morphology and damage mitochondria, cell membranes, and vacuoles. The results of molecular docking of compound U22 and pydiflumetofen with succinate dehydrogenase (SDH) indicated they interact well with the active site of SDH. This study validated our approach and design strategy to produce compounds with an enhanced biological activity as compared to the parent structure.
中文翻译:

发现针对琥珀酸脱氢酶的高效新型抗真菌先导化合物:带有 N-苯基取代酰胺片段的吡唑-4-甲酰胺衍生物
开发环境友好型杀菌剂对于解决农药耐药性上升的问题至关重要。本研究设计并合成了一系列含有N-苯基取代酰胺片段的新型吡唑-4-甲酰胺衍生物。目标化合物的结构经1 H NMR、 13 C NMR、HRMS 确证,最活泼化合物的晶体结构为N -(1-(4-(4-(叔丁基)苯甲酰胺基)苯基)丙-通过X射线单晶衍射进一步测定2-基)-3-(二氟甲基) -N-甲氧基-1-甲基-1H-吡唑-4-甲酰胺( U22 )。生物测定结果表明,26个目标化合物对核盘菌具有良好的体外抗真菌活性,化合物U12 、 U13 、 U15 、 U16 、 U18 、 U22和U23的EC 50值分别为4.17±0.46、8.04±0.71、7.01±0.71。分别为 12.77 ± 1.00、8.11 ± 0.70、0.94 ± 0.11 和 9.48 ± 0.83 μg·mL –1 ,与对照联苯吡菌胺 (6.70 ± 0.47 μg·mL –1 )、fluxapyroxad (0.71 ± 0.14 μg·mL –1 ) 相似。 mL –1 ) 和吡虫螨酯 (0.06 ± 0.01 μg·mL –1 )。此外,针对核盘菌的体内抗真菌活性结果表明,化合物U12 (80.6%)和U22 (89.9%)在200 μg·mL –1浓度下具有优异的预防效果,与对照吡虫螨酯(82.4%)相同。 扫描电镜和透射电镜研究发现,化合物U22可以破坏菌丝形态,损伤线粒体、细胞膜和液泡。化合物U22和pydiflumetofen与琥珀酸脱氢酶(SDH)的分子对接结果表明它们与SDH的活性位点有良好的相互作用。这项研究验证了我们的方法和设计策略,以生产与母体结构相比具有增强生物活性的化合物。
更新日期:2023-11-29
中文翻译:

发现针对琥珀酸脱氢酶的高效新型抗真菌先导化合物:带有 N-苯基取代酰胺片段的吡唑-4-甲酰胺衍生物
开发环境友好型杀菌剂对于解决农药耐药性上升的问题至关重要。本研究设计并合成了一系列含有N-苯基取代酰胺片段的新型吡唑-4-甲酰胺衍生物。目标化合物的结构经1 H NMR、 13 C NMR、HRMS 确证,最活泼化合物的晶体结构为N -(1-(4-(4-(叔丁基)苯甲酰胺基)苯基)丙-通过X射线单晶衍射进一步测定2-基)-3-(二氟甲基) -N-甲氧基-1-甲基-1H-吡唑-4-甲酰胺( U22 )。生物测定结果表明,26个目标化合物对核盘菌具有良好的体外抗真菌活性,化合物U12 、 U13 、 U15 、 U16 、 U18 、 U22和U23的EC 50值分别为4.17±0.46、8.04±0.71、7.01±0.71。分别为 12.77 ± 1.00、8.11 ± 0.70、0.94 ± 0.11 和 9.48 ± 0.83 μg·mL –1 ,与对照联苯吡菌胺 (6.70 ± 0.47 μg·mL –1 )、fluxapyroxad (0.71 ± 0.14 μg·mL –1 ) 相似。 mL –1 ) 和吡虫螨酯 (0.06 ± 0.01 μg·mL –1 )。此外,针对核盘菌的体内抗真菌活性结果表明,化合物U12 (80.6%)和U22 (89.9%)在200 μg·mL –1浓度下具有优异的预防效果,与对照吡虫螨酯(82.4%)相同。 扫描电镜和透射电镜研究发现,化合物U22可以破坏菌丝形态,损伤线粒体、细胞膜和液泡。化合物U22和pydiflumetofen与琥珀酸脱氢酶(SDH)的分子对接结果表明它们与SDH的活性位点有良好的相互作用。这项研究验证了我们的方法和设计策略,以生产与母体结构相比具有增强生物活性的化合物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号