当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Two-Component Alkenyl Catellani Reaction for the Construction of C—N Axial Chirality†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-11-29 , DOI: 10.1002/cjoc.202300621 Chenggui Wu 1, 2 , Ze‐Shui Liu 1 , Yong Shang 1 , Chang Liu 1 , Shuang Deng 3 , Hong‐Gang Cheng 1 , Hengjiang Cong 1 , Yinchun Jiao 3 , Qianghui Zhou 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2023-11-29 , DOI: 10.1002/cjoc.202300621 Chenggui Wu 1, 2 , Ze‐Shui Liu 1 , Yong Shang 1 , Chang Liu 1 , Shuang Deng 3 , Hong‐Gang Cheng 1 , Hengjiang Cong 1 , Yinchun Jiao 3 , Qianghui Zhou 1
Affiliation
|
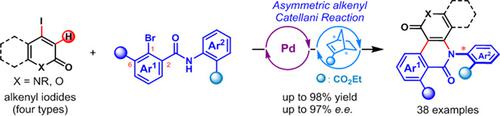
Herein, we report an asymmetric two-component alkenyl Catellani reaction for the construction of C—N axial chirality through a palladium/chiral norbornene cooperative catalysis and an axial-to-axial chirality transfer process. Various partially aromatic iodinated 2-pyridones, quinolones, coumarin and uracil substrates react with 2,6-disubstituted aryl bromides with a tethered amide group, to afford a wide variety of polycyclic C—N atropisomers (38 examples, up to 97% e.e.). The obtained C—N axial chirality originates from the preformed transient C—C axial chirality with high fidelity. The synthetic utility of this chemistry is demonstrated by facile preparation of complex quinoline and pyridine based C—N atropisomers through a N-deprotection and aromatization sequence. In addition, a remote axial-to-central diastereoinduction process dictated by C—N axial chirality is observed with excellent diastereocontrol.
中文翻译:

用于构建 C—N 轴向手性的不对称双组分烯基 Catellani 反应†
在此,我们报道了一种不对称双组分烯基Catellani反应,通过钯/手性降冰片烯协同催化和轴间手性传递过程构建CN轴手性。各种部分芳香族碘化 2-吡啶酮、喹诺酮、香豆素和尿嘧啶底物与带有束缚酰胺基团的 2,6-二取代芳基溴反应,得到多种多环 CN 阻转异构体(38 个实例,高达 97% ee) 。所获得的CN轴向手性源于预先形成的高保真度瞬态CC轴向手性。通过 N-脱保护和芳构化序列轻松制备基于喹啉和吡啶的 C-N 阻转异构体,证明了该化学的合成效用。此外,通过出色的非对映控制,观察到由 CN 轴向手性决定的远程轴向到中心非对映诱导过程。
更新日期:2023-11-29

中文翻译:

用于构建 C—N 轴向手性的不对称双组分烯基 Catellani 反应†
在此,我们报道了一种不对称双组分烯基Catellani反应,通过钯/手性降冰片烯协同催化和轴间手性传递过程构建CN轴手性。各种部分芳香族碘化 2-吡啶酮、喹诺酮、香豆素和尿嘧啶底物与带有束缚酰胺基团的 2,6-二取代芳基溴反应,得到多种多环 CN 阻转异构体(38 个实例,高达 97% ee) 。所获得的CN轴向手性源于预先形成的高保真度瞬态CC轴向手性。通过 N-脱保护和芳构化序列轻松制备基于喹啉和吡啶的 C-N 阻转异构体,证明了该化学的合成效用。此外,通过出色的非对映控制,观察到由 CN 轴向手性决定的远程轴向到中心非对映诱导过程。



















































 京公网安备 11010802027423号
京公网安备 11010802027423号