当前位置:
X-MOL 学术
›
J. Pharm. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Apatinib and gamabufotalin co-loaded lipid/Prussian blue nanoparticles for synergistic therapy to gastric cancer with metastasis
Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2023-11-29 , DOI: 10.1016/j.jpha.2023.11.011 Binlong Chen 1, 2 , Yanzhong Zhao 1 , Zichang Lin 3 , Jiahao Liang 4 , Jialong Fan 4 , Yanyan Huang 1 , Leye He 2 , Bin Liu 4
Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2023-11-29 , DOI: 10.1016/j.jpha.2023.11.011 Binlong Chen 1, 2 , Yanzhong Zhao 1 , Zichang Lin 3 , Jiahao Liang 4 , Jialong Fan 4 , Yanyan Huang 1 , Leye He 2 , Bin Liu 4
Affiliation
|
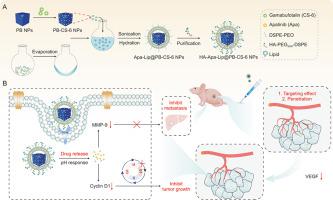
Due to the non-targeted release and low solubility of anti-gastric cancer agent, apatinib (Apa), a first-line drug with long-term usage in a high dosage often induces multi-drug resistance and causes serious side effects. In order to avoid these drawbacks, lipid-film-coated Prussian blue nanoparticles (PB NPs) with hyaluronan (HA) modification was used for Apa loading to improve its solubility and targeting ability. Furthermore, anti-tumor compound of gamabufotalin (CS-6) was selected as a partner of Apa with reducing dosage for combinational gastric therapy. Thus, HA-Apa-Lip@PB-CS-6 NPs were constructed to synchronously transport the two drugs into tumor tissue. assay indicated that HA-Apa-Lip@PB-CS-6 NPs can synergistically inhibit proliferation and invasion/metastasis of BGC-823 cells via downregulating vascular endothelial growth factor receptor (VEGFR) and matrix metalloproteinase-9 (MMP-9). assay demonstrated strongest anti-tumor growth and liver metastasis of HA-Apa-Lip@PB-CS-6 NPs administration in BGC-823 cells-bearing mice compared with other groups due to the excellent penetration in tumor tissues and outstanding synergistic effects. In summary, we have successfully developed a new nanocomplexes for synchronous Apa/CS-6 delivery and synergistic gastric cancer (GC) therapy.
中文翻译:

阿帕替尼和加布福他林共载脂质/普鲁士蓝纳米粒子协同治疗转移性胃癌
由于抗胃癌药物的非靶向释放和溶解度低,一线药物阿帕替尼(Apa)长期大剂量使用往往会诱发多重耐药,产生严重的副作用。为了避免这些缺点,采用透明质酸(HA)修饰的脂质膜包覆的普鲁士蓝纳米粒子(PB NPs)用于Apa负载,以提高其溶解度和靶向能力。此外,选择抗肿瘤化合物伽布福林(CS-6)作为Apa的搭档,减少联合胃治疗的剂量。因此,构建了HA-Apa-Lip@PB-CS-6 NPs以将两种药物同步转运到肿瘤组织中。实验表明,HA-Apa-Lip@PB-CS-6 NPs可以通过下调血管内皮生长因子受体(VEGFR)和基质金属蛋白酶-9(MMP-9)协同抑制BGC-823细胞的增殖和侵袭/转移。实验表明,与其他组相比,HA-Apa-Lip@PB-CS-6 NPs 在 BGC-823 细胞荷载小鼠中的给药具有最强的抗肿瘤生长和肝转移作用,因为其在肿瘤组织中具有良好的渗透性和突出的协同作用。总之,我们成功开发了一种用于同步 Apa/CS-6 递送和协同胃癌 (GC) 治疗的新型纳米复合物。
更新日期:2023-11-29
中文翻译:

阿帕替尼和加布福他林共载脂质/普鲁士蓝纳米粒子协同治疗转移性胃癌
由于抗胃癌药物的非靶向释放和溶解度低,一线药物阿帕替尼(Apa)长期大剂量使用往往会诱发多重耐药,产生严重的副作用。为了避免这些缺点,采用透明质酸(HA)修饰的脂质膜包覆的普鲁士蓝纳米粒子(PB NPs)用于Apa负载,以提高其溶解度和靶向能力。此外,选择抗肿瘤化合物伽布福林(CS-6)作为Apa的搭档,减少联合胃治疗的剂量。因此,构建了HA-Apa-Lip@PB-CS-6 NPs以将两种药物同步转运到肿瘤组织中。实验表明,HA-Apa-Lip@PB-CS-6 NPs可以通过下调血管内皮生长因子受体(VEGFR)和基质金属蛋白酶-9(MMP-9)协同抑制BGC-823细胞的增殖和侵袭/转移。实验表明,与其他组相比,HA-Apa-Lip@PB-CS-6 NPs 在 BGC-823 细胞荷载小鼠中的给药具有最强的抗肿瘤生长和肝转移作用,因为其在肿瘤组织中具有良好的渗透性和突出的协同作用。总之,我们成功开发了一种用于同步 Apa/CS-6 递送和协同胃癌 (GC) 治疗的新型纳米复合物。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号