当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 3-hydroxy-2-naphthohydrazide-based hydrazones and their implications in diabetic management via in vitro and in silico approaches
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-11-27 , DOI: 10.1002/ardp.202300544 Mussarat Tasleem 1 , Saeed Ullah 2 , Sobia Ahsan Halim 2 , Ifra Urooj 1 , Nadeem Ahmed 1 , Rabia Munir 1 , Ajmal Khan 2 , Attalla F El-Kott 3, 4 , Parham Taslimi 5 , Sally Negm 6 , Ahmed Al-Harrasi 2 , Zahid Shafiq 1, 7
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2023-11-27 , DOI: 10.1002/ardp.202300544 Mussarat Tasleem 1 , Saeed Ullah 2 , Sobia Ahsan Halim 2 , Ifra Urooj 1 , Nadeem Ahmed 1 , Rabia Munir 1 , Ajmal Khan 2 , Attalla F El-Kott 3, 4 , Parham Taslimi 5 , Sally Negm 6 , Ahmed Al-Harrasi 2 , Zahid Shafiq 1, 7
Affiliation
|
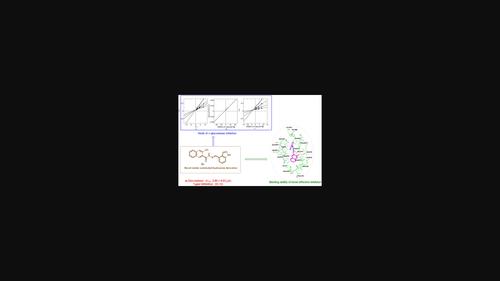
Diabetes mellitus (DM) has prevailed as a chronic health condition and has become a serious global health issue due to its numerous consequences and high prevalence. We have synthesized a series of hydrazone derivatives and tested their antidiabetic potential by inhibiting the essential carbohydrate catabolic enzyme, “α-glucosidase.” Several approaches including fourier transform infrared, 1H NMR, and 13C NMR were utilized to confirm the structures of all the synthesized derivatives. In vitro analysis of compounds 3a–3p displayed more effective inhibitory activities against α-glucosidase with IC50 in a range of 2.80–29.66 µM as compared with the commercially available inhibitor, acarbose (IC50 = 873.34 ± 1.67 M). Compound 3h showed the highest inhibitory potential with an IC50 value of 2.80 ± 0.03 µM, followed by 3i (IC50 = 4.13 ± 0.06 µM), 3f (IC50 = 5.18 ± 0.10 µM), 3c (IC50 = 5.42 ± 0.11 µM), 3g (IC50 = 6.17 ± 0.15 µM), 3d (IC50 = 6.76 ± 0.20 µM), 3a (IC50 = 9.59 ± 0.14 µM), and 3n (IC50 = 10.01 ± 0.42 µM). Kinetics analysis of the most potent compound 3h revealed a concentration-dependent form of inhibition by 3h with Ki value = 4.76 ± 0.0068 µM. Additionally, an in silico docking approach was applied to predict the binding patterns of all the compounds, which indicates that the hydrazide and the naphthalene-ol groups play a vital role in the binding of the compounds with the essential residues (i.e., Glu277 and Gln279) of the α-glucosidase enzyme.
中文翻译:

3-羟基-2-萘甲酰肼基腙的合成及其在体外和计算机模拟方法中对糖尿病治疗的影响
糖尿病(DM)作为一种慢性健康状况普遍存在,并且由于其多种后果和高患病率而成为一个严重的全球健康问题。我们合成了一系列腙衍生物,并通过抑制必需的碳水化合物分解代谢酶“α-葡萄糖苷酶”来测试其抗糖尿病潜力。采用傅里叶变换红外、 1 H NMR 和13 C NMR 等多种方法来确认所有合成衍生物的结构。与市售抑制剂阿卡波糖相比,化合物3a – 3p的体外分析显示出更有效的 α-葡萄糖苷酶抑制活性,IC 50在 2.80–29.66 µM 范围内 (IC 50 = 873.34 ± 1.67 M)。化合物3h显示出最高的抑制潜力,IC 50值为 2.80 ± 0.03 µM,其次是3i (IC 50 = 4.13 ± 0.06 µM)、 3f (IC 50 = 5.18 ± 0.10 µM)、 3c (IC 50 = 5.42 ± 0.11) µM)、 3g (IC 50 = 6.17 ± 0.15 µM)、 3d (IC 50 = 6.76 ± 0.20 µM)、 3a (IC 50 = 9.59 ± 0.14 µM) 和3n (IC 50 = 10.01 ± 0.42 µM)。最有效的化合物3 小时的动力学分析显示, 3 小时后呈浓度依赖性抑制形式, K i值 = 4.76 ± 0.0068 µM。 此外,应用计算机对接方法来预测所有化合物的结合模式,这表明酰肼和萘酚基团在化合物与必需残基(即 Glu277 和 Gln279)的结合中发挥着至关重要的作用。 )的α-葡萄糖苷酶。
更新日期:2023-11-27
中文翻译:

3-羟基-2-萘甲酰肼基腙的合成及其在体外和计算机模拟方法中对糖尿病治疗的影响
糖尿病(DM)作为一种慢性健康状况普遍存在,并且由于其多种后果和高患病率而成为一个严重的全球健康问题。我们合成了一系列腙衍生物,并通过抑制必需的碳水化合物分解代谢酶“α-葡萄糖苷酶”来测试其抗糖尿病潜力。采用傅里叶变换红外、 1 H NMR 和13 C NMR 等多种方法来确认所有合成衍生物的结构。与市售抑制剂阿卡波糖相比,化合物3a – 3p的体外分析显示出更有效的 α-葡萄糖苷酶抑制活性,IC 50在 2.80–29.66 µM 范围内 (IC 50 = 873.34 ± 1.67 M)。化合物3h显示出最高的抑制潜力,IC 50值为 2.80 ± 0.03 µM,其次是3i (IC 50 = 4.13 ± 0.06 µM)、 3f (IC 50 = 5.18 ± 0.10 µM)、 3c (IC 50 = 5.42 ± 0.11) µM)、 3g (IC 50 = 6.17 ± 0.15 µM)、 3d (IC 50 = 6.76 ± 0.20 µM)、 3a (IC 50 = 9.59 ± 0.14 µM) 和3n (IC 50 = 10.01 ± 0.42 µM)。最有效的化合物3 小时的动力学分析显示, 3 小时后呈浓度依赖性抑制形式, K i值 = 4.76 ± 0.0068 µM。 此外,应用计算机对接方法来预测所有化合物的结合模式,这表明酰肼和萘酚基团在化合物与必需残基(即 Glu277 和 Gln279)的结合中发挥着至关重要的作用。 )的α-葡萄糖苷酶。































 京公网安备 11010802027423号
京公网安备 11010802027423号