当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Volatile Methyl Siloxane Atmospheric Oxidation Mechanism from a Theoretical Perspective─How is the Siloxanol Formed?
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-11-27 , DOI: 10.1021/acs.jpca.3c06287 Mitchell W Alton 1, 2 , Virginia L Johnson 1 , Sandeep Sharma 1 , Eleanor C Browne 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2023-11-27 , DOI: 10.1021/acs.jpca.3c06287 Mitchell W Alton 1, 2 , Virginia L Johnson 1 , Sandeep Sharma 1 , Eleanor C Browne 1, 2
Affiliation
|
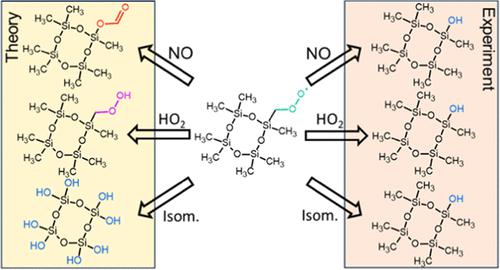
Despite several investigations on the atmospheric fate of cyclic volatile methyl siloxanes (VMS), the oxidation chemistry of these purely anthropogenic, high production volume compounds is poorly understood. This led to uncertainties in the environmental impact and fate of the oxidation products. According to laboratory measurements, the main VMS oxidation product is the siloxanol (a −CH3 replaced with an −OH); however, none of the mechanisms proposed to date satisfactorily explain its formation. Motivated by our previous experimental observations of VMS oxidation products, we use theoretical quantum chemical calculations to (1) explore a previously unconsidered reaction pathway to form the siloxanol from a reaction of a siloxy radical with gas-phase water, (2) investigate differences in reaction rates of radical intermediates in hexamethylcyclotrisiloxane (D3) and octamethylcyclotetrasiloxane (D4) oxidation, and (3) attempt to explain the experimentally observed products. Our results suggest that while the proposed reaction of the siloxy radical with water to form the siloxanol can occur, it is too slow to compete with other unimolecular reactions and thus cannot explain the observed siloxanol formation. We also find that the reaction between the initial D3 peroxy radical (RO2•) with HO2• is slower than previously anticipated (calculated as 3 × 10–13 cm3 molecule–1 s–1 for D3 and 2 × 10–11 cm3 molecule–1 s–1 for D4 compared to the general rate of ∼1 × 10–11 cm3 molecule–1 s–1). Finally, we compare the anticipated fates of the RO2• under a variety of conditions and find that a reaction with NO (assuming a general RO2• + NO bimolecular rate constant of 9 × 10–12 cm3 molecule–1 s–1) will likely be the dominant fate in urban conditions, while isomerization can be important in cleaner environments.
中文翻译:

从理论角度探讨挥发性甲基硅氧烷的大气氧化机理─硅氧烷醇是如何形成的?
尽管对环状挥发性甲基硅氧烷(VMS)在大气中的归宿进行了多项研究,但人们对这些纯人为的高产量化合物的氧化化学知之甚少。这导致了环境影响和氧化产物的命运的不确定性。根据实验室测量,主要的VMS氧化产物是硅氧烷醇(-CH 3被-OH取代);然而,迄今为止提出的机制均未令人满意地解释其形成。受我们之前对 VMS 氧化产物实验观察的启发,我们使用理论量子化学计算来 (1) 探索以前未考虑的反应途径,通过硅氧基自由基与气相水的反应形成硅氧烷醇,(2) 研究六甲基环三硅氧烷(D3)和八甲基环四硅氧烷(D4)氧化中自由基中间体的反应速率,以及(3)尝试解释实验观察到的产物。我们的结果表明,虽然所提出的硅氧基自由基与水形成硅氧烷醇的反应可以发生,但它太慢而无法与其他单分子反应竞争,因此无法解释观察到的硅氧烷醇的形成。 我们还发现初始 D3 过氧自由基 (RO 2 • ) 与 HO 2 •之间的反应比之前预期的要慢(计算为 D3 为 3 × 10 –13 cm 3分子–1 s –1和 2 × 10 –11 cm 3分子–1 s –1对于 D4 而言,一般速率为 ∼1 × 10 –11 cm 3分子–1 s –1 )。最后,我们比较了 RO 2 •在各种条件下的预期命运,发现与 NO 的反应(假设一般 RO 2 • + NO 双分子速率常数为 9 × 10 –12 cm 3分子–1 s –1 )可能是城市条件下的主要命运,而异构化在更清洁的环境中可能很重要。
更新日期:2023-11-27
中文翻译:

从理论角度探讨挥发性甲基硅氧烷的大气氧化机理─硅氧烷醇是如何形成的?
尽管对环状挥发性甲基硅氧烷(VMS)在大气中的归宿进行了多项研究,但人们对这些纯人为的高产量化合物的氧化化学知之甚少。这导致了环境影响和氧化产物的命运的不确定性。根据实验室测量,主要的VMS氧化产物是硅氧烷醇(-CH 3被-OH取代);然而,迄今为止提出的机制均未令人满意地解释其形成。受我们之前对 VMS 氧化产物实验观察的启发,我们使用理论量子化学计算来 (1) 探索以前未考虑的反应途径,通过硅氧基自由基与气相水的反应形成硅氧烷醇,(2) 研究六甲基环三硅氧烷(D3)和八甲基环四硅氧烷(D4)氧化中自由基中间体的反应速率,以及(3)尝试解释实验观察到的产物。我们的结果表明,虽然所提出的硅氧基自由基与水形成硅氧烷醇的反应可以发生,但它太慢而无法与其他单分子反应竞争,因此无法解释观察到的硅氧烷醇的形成。 我们还发现初始 D3 过氧自由基 (RO 2 • ) 与 HO 2 •之间的反应比之前预期的要慢(计算为 D3 为 3 × 10 –13 cm 3分子–1 s –1和 2 × 10 –11 cm 3分子–1 s –1对于 D4 而言,一般速率为 ∼1 × 10 –11 cm 3分子–1 s –1 )。最后,我们比较了 RO 2 •在各种条件下的预期命运,发现与 NO 的反应(假设一般 RO 2 • + NO 双分子速率常数为 9 × 10 –12 cm 3分子–1 s –1 )可能是城市条件下的主要命运,而异构化在更清洁的环境中可能很重要。















































 京公网安备 11010802027423号
京公网安备 11010802027423号