当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chasing the CMD/AMLA Mechanism C–H···Pd Interactions in the Cyclopalladation Reaction of N,N-Dimethylbenzylamine with Pd(OAc)2
Organometallics ( IF 2.5 ) Pub Date : 2023-11-27 , DOI: 10.1021/acs.organomet.3c00208 M. Arif Sajjad 1 , Peter Schwerdtfeger 2 , Patrick J. B. Edwards 3 , John A. Harrison 4 , Alastair J. Nielson 4
Organometallics ( IF 2.5 ) Pub Date : 2023-11-27 , DOI: 10.1021/acs.organomet.3c00208 M. Arif Sajjad 1 , Peter Schwerdtfeger 2 , Patrick J. B. Edwards 3 , John A. Harrison 4 , Alastair J. Nielson 4
Affiliation
|
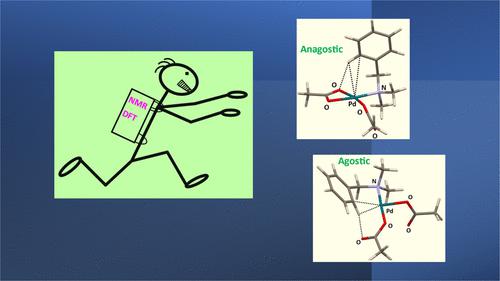
700 MHz 1H NMR spectral examination of the reaction between Me2NCH2Ph and Pd(OAc)2 in CDCl3 reveals that several anagostic complexes form, but an agostic intermediate is not identified by way of a lowered 1JC–H coupling constant. Density functional theory (DFT) calculations were used to compare characteristic features of the putative agostic intermediate when the acetato ligand C═O···H–C interaction is present as in the CMD/AMLA mechanism and when it is not. With the C═O···H–C interaction present, the agostic C–H bond is significantly longer, the H and C atoms are closer to the metal, natural bond orbital (NBO) analysis indicates that agostic C–Hσ orbital donation to Pd-based orbitals is significantly greater, π-syndetic donation is similar, and coulombic attraction between the agostic carbon and the Pd center is greater as is the repulsion between the H and Pd atoms. Whether the C═O···H–C interaction is present or not, the electron withdrawal substituents SO2Cl and NO2 at the para position of the ligand on energy minimization reduce the effect of the interaction, whereas the electron-donating groups B(OH)3– and NMe2 give η1-σ-lone pair donor complexes and S– produces a Pd–C–H σ-bond. Overall, the presence of the C═O···H–C interaction enhances the agostic interaction produced during the reaction of the N,N-dimethylbenzylamine with Pd(OAc)2, which gives credence to the importance of the AMLA mechanism.
中文翻译:

N,N-二甲基苄胺与 Pd(OAc)2 环钯化反应中 C-H·Pd 相互作用的探究
对 Me 2 NCH 2 Ph 和 Pd(OAc) 2在 CDCl 3中的反应进行700 MHz 1 H NMR 光谱检查表明,形成了几种抑制复合物,但通过降低的1 J C-H 耦合并未识别出抑制中间体持续的。使用密度泛函理论 (DFT) 计算来比较当 CMD/AMLA 机制中存在乙酰基配体 C=O…H-C 相互作用时和不存在时,假定的激动中间体的特征。由于存在C=O···H–C相互作用,不规则的C–H键明显更长,H和C原子更接近金属,自然键轨道(NBO)分析表明不规则的C–Hσ轨道捐赠与 Pd 基轨道的关系明显更大,π-合成捐赠相似,并且远古碳和 Pd 中心之间的库仑吸引力更大,H 和 Pd 原子之间的排斥力也更大。无论是否存在C=O···H-C相互作用,配体对位的吸电子取代基SO 2 Cl和NO 2导致能量最小化,降低了相互作用的影响,而给电子基团B(OH) 3 –和 NMe 2形成 η 1 -σ-孤电子对供体配合物,S –产生 Pd–C–H σ-键。总体而言,C=O…H-C相互作用的存在增强了N , N-二甲基苄胺与Pd(OAc) 2反应过程中产生的激动相互作用,这证实了AMLA机制的重要性。
更新日期:2023-11-27
中文翻译:

N,N-二甲基苄胺与 Pd(OAc)2 环钯化反应中 C-H·Pd 相互作用的探究
对 Me 2 NCH 2 Ph 和 Pd(OAc) 2在 CDCl 3中的反应进行700 MHz 1 H NMR 光谱检查表明,形成了几种抑制复合物,但通过降低的1 J C-H 耦合并未识别出抑制中间体持续的。使用密度泛函理论 (DFT) 计算来比较当 CMD/AMLA 机制中存在乙酰基配体 C=O…H-C 相互作用时和不存在时,假定的激动中间体的特征。由于存在C=O···H–C相互作用,不规则的C–H键明显更长,H和C原子更接近金属,自然键轨道(NBO)分析表明不规则的C–Hσ轨道捐赠与 Pd 基轨道的关系明显更大,π-合成捐赠相似,并且远古碳和 Pd 中心之间的库仑吸引力更大,H 和 Pd 原子之间的排斥力也更大。无论是否存在C=O···H-C相互作用,配体对位的吸电子取代基SO 2 Cl和NO 2导致能量最小化,降低了相互作用的影响,而给电子基团B(OH) 3 –和 NMe 2形成 η 1 -σ-孤电子对供体配合物,S –产生 Pd–C–H σ-键。总体而言,C=O…H-C相互作用的存在增强了N , N-二甲基苄胺与Pd(OAc) 2反应过程中产生的激动相互作用,这证实了AMLA机制的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号