当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bicyclopentylation of Alcohols with Thianthrenium Reagents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-27 , DOI: 10.1021/jacs.3c10024 Zibo Bai 1 , Beatrice Lansbergen 1 , Tobias Ritter 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-27 , DOI: 10.1021/jacs.3c10024 Zibo Bai 1 , Beatrice Lansbergen 1 , Tobias Ritter 1
Affiliation
|
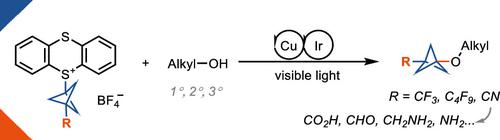
Herein we present the first method for the synthesis of bicyclo[1.1.1]pentyl (BCP) alkyl ethers from alcohols. The reaction uses BCP–thianthrenium reagents and is catalyzed by a dual copper/photoredox catalyst system. Unlike known alkylations of tertiary alcohols via carbocation intermediates, our Cu-mediated radical process circumvents the labile BCP carbocations. The approach demonstrates a broad tolerance for functional groups when applied to primary, secondary, and even tertiary alcohols. In addition, we highlight the utility of this method in late-stage functionalizations of both natural products and pharmaceuticals as well as in the rapid construction of BCP analogs of known pharmaceuticals that would otherwise be difficult to access.
中文翻译:

用铊试剂对醇进行双环戊基化
在此,我们提出了第一种由醇合成双环[1.1.1]戊基(BCP)烷基醚的方法。该反应使用 BCP-铊试剂,并由双铜/光氧化还原催化剂系统催化。与已知的通过碳阳离子中间体进行的叔醇烷基化不同,我们的 Cu 介导的自由基过程规避了不稳定的 BCP 碳阳离子。该方法在应用于伯醇、仲醇甚至叔醇时表现出对官能团的广泛耐受性。此外,我们还强调了该方法在天然产物和药物的后期功能化以及快速构建已知药物的 BCP 类似物中的效用,否则这些方法将很难获得。
更新日期:2023-11-27
中文翻译:

用铊试剂对醇进行双环戊基化
在此,我们提出了第一种由醇合成双环[1.1.1]戊基(BCP)烷基醚的方法。该反应使用 BCP-铊试剂,并由双铜/光氧化还原催化剂系统催化。与已知的通过碳阳离子中间体进行的叔醇烷基化不同,我们的 Cu 介导的自由基过程规避了不稳定的 BCP 碳阳离子。该方法在应用于伯醇、仲醇甚至叔醇时表现出对官能团的广泛耐受性。此外,我们还强调了该方法在天然产物和药物的后期功能化以及快速构建已知药物的 BCP 类似物中的效用,否则这些方法将很难获得。































 京公网安备 11010802027423号
京公网安备 11010802027423号