当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Cu/CeO2 for Ketonization of Carboxylic Acids with Synergistic Interactions
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-11-27 , DOI: 10.1021/acs.iecr.3c03237
Shan Zhang 1 , Binbo Jiang 1 , Mingliang Tong 1 , Yao Yang 1 , Zuwei Liao 1 , Zhengliang Huang 1 , Jingyuan Sun 1 , Jingdai Wang 1 , Yongrong Yang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-11-27 , DOI: 10.1021/acs.iecr.3c03237
Shan Zhang 1 , Binbo Jiang 1 , Mingliang Tong 1 , Yao Yang 1 , Zuwei Liao 1 , Zhengliang Huang 1 , Jingyuan Sun 1 , Jingdai Wang 1 , Yongrong Yang 1
Affiliation
|
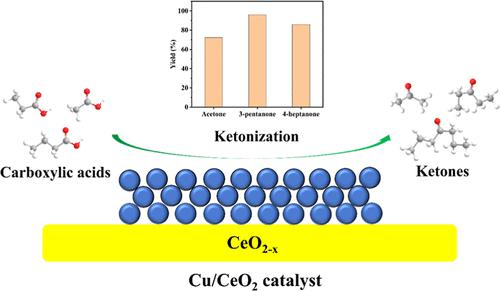
Cost-effective heterogeneous catalysts are critical to the ketonization of carboxylic acids for producing biofuels and platform chemicals. Herein, nanocrystalline Cu-loaded CeO2 with 1.0–9.0 wt % Cu loadings was synthesized by the precipitation-impregnation method, in which 3.0 wt % Cu/CeO2 exhibits the best performance of 72% acetone yield at 330 °C, 97% 3-pentanone yield at 360 °C, and 86% 4-heptanone yield at 400 °C, with good durability up to 10 h on stream. Detailed analyses reveal that 3.0 wt % Cu/CeO2 has the highest percentage of O-vacancy sites, more moderate acid–base sites, and better capacity to adsorb and activate carboxylic acids. In situ DRIFT spectra indicate that monodentate, bridging bidentate, and chelating bidentate modes coexist when carboxylic acid is dissociatively adsorbed on 3.0 wt % Cu/CeO2. Based on the above investigations, we propose a mechanism over 3.0 wt % Cu/CeO2 through the β-ketoacid intermediate route, which gives more details to help future research into the design and development of catalysts for ketonization.
中文翻译:

具有协同相互作用的高效 Cu/CeO2 用于羧酸酮化
具有成本效益的多相催化剂对于生产生物燃料和平台化学品的羧酸酮化至关重要。本文通过沉淀-浸渍法合成了Cu负载量为1.0-9.0 wt %的纳米晶Cu负载CeO 2 ,其中3.0 wt % Cu/CeO 2表现出最佳性能,在330 °C时丙酮产率为72%,97% 360 °C 时 3-戊酮收率,400 °C 时 4-庚酮收率 86%,在运行长达 10 小时内具有良好的耐久性。详细分析表明,3.0 wt% Cu/CeO 2具有最高百分比的O空位位点、更温和的酸碱位点以及更好的吸附和活化羧酸的能力。原位DRIFT光谱表明,当羧酸解离吸附在3.0wt%Cu/CeO 2上时,单齿、桥联双齿和螯合双齿模式共存。基于上述研究,我们提出了通过β-酮酸中间体途径获得超过3.0 wt% Cu/CeO 2的机理,这为未来酮化催化剂的设计和开发研究提供了更多细节。
更新日期:2023-11-27
中文翻译:

具有协同相互作用的高效 Cu/CeO2 用于羧酸酮化
具有成本效益的多相催化剂对于生产生物燃料和平台化学品的羧酸酮化至关重要。本文通过沉淀-浸渍法合成了Cu负载量为1.0-9.0 wt %的纳米晶Cu负载CeO 2 ,其中3.0 wt % Cu/CeO 2表现出最佳性能,在330 °C时丙酮产率为72%,97% 360 °C 时 3-戊酮收率,400 °C 时 4-庚酮收率 86%,在运行长达 10 小时内具有良好的耐久性。详细分析表明,3.0 wt% Cu/CeO 2具有最高百分比的O空位位点、更温和的酸碱位点以及更好的吸附和活化羧酸的能力。原位DRIFT光谱表明,当羧酸解离吸附在3.0wt%Cu/CeO 2上时,单齿、桥联双齿和螯合双齿模式共存。基于上述研究,我们提出了通过β-酮酸中间体途径获得超过3.0 wt% Cu/CeO 2的机理,这为未来酮化催化剂的设计和开发研究提供了更多细节。

































 京公网安备 11010802027423号
京公网安备 11010802027423号