当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel diamine non-aqueous absorbent based on N-methyl diethanolamine regulation for energy-efficient CO2 capture
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2023-11-29 , DOI: 10.1039/d3re00506b Zhen Wang 1 , Zhitao Han 1 , Xiao Yang 1 , Zelu Zhou 1 , Xi Wu 1 , Song Zhou 2 , Shijian Lu 3
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2023-11-29 , DOI: 10.1039/d3re00506b Zhen Wang 1 , Zhitao Han 1 , Xiao Yang 1 , Zelu Zhou 1 , Xi Wu 1 , Song Zhou 2 , Shijian Lu 3
Affiliation
|
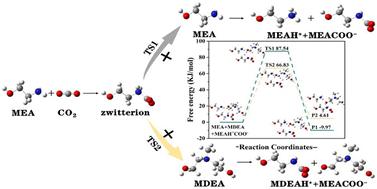
Non-aqueous absorbents receive more and more attention due to their low sensible heat and evaporation latent heat. But their low CO2 desorption efficiency and high desorption reaction heat are still major limits for practical application. Herein, a novel non-aqueous diamine absorbent based on alkanolamine regulation is proposed. In the experiments, monoethanolamine (MEA) was used as the main absorbent for ensuring high CO2 absorption capacity and absorption rate, while polyethylene glycol 200 (PEG200) was used as a cosolvent to reduce evaporation latent heat. Subsequently, MEA/PEG200 non-aqueous absorbents were composed with three typical secondary/tertiary amines including N-methyldiethanolamine (MDEA), diethanolamine (DEA) and triethanolamine (TEA), respectively, and their regulation effects on CO2 capture performance were investigated systematically. The results showed that the MEA/MDEA/PEG non-aqueous system exhibited a superior CO2 desorption performance. The corresponding maximum regeneration efficiency reached up to 82.1%, which was significantly higher than the case without addition of MDEA. It still kept a high CO2 desorption efficiency (79%) after the 8th regeneration cycle. According to thermodynamic analysis, the desorption reaction heat for the MEA/MDEA/PEG non-aqueous system was only 1.40 GJ per ton CO2, which was 20% lower than the non-aqueous MEA/PEG200 absorbent. And its total regeneration energy consumption was 1.90 GJ per ton CO2, which was reduced by 48.1% compared to MEA aqueous solution. FT-IR, 13C-NMR and DFT calculations indicated that the introduction of the MDEA regulator would result in a much lower reaction energy barrier between zwitterions and MDEA compared to that between zwitterions and MEA. Besides, it would induce the formation of protonated MDEA (MDEAH+), which was much easier for MDEA regeneration.
中文翻译:

一种基于 N-甲基二乙醇胺调节的新型二胺非水吸收剂,用于高效节能的二氧化碳捕获
非水吸收剂由于其较低的显热和蒸发潜热而受到越来越多的关注。但其低CO 2解吸效率和高解吸反应热仍然是实际应用的主要限制。在此,提出了一种基于烷醇胺调节的新型非水二胺吸收剂。实验中,采用单乙醇胺(MEA)作为主要吸收剂,以保证较高的CO 2吸收能力和吸收率,同时采用聚乙二醇200(PEG200)作为助溶剂,以降低蒸发潜热。随后,分别与N-甲基二乙醇胺(MDEA)、二乙醇胺(DEA)和三乙醇胺(TEA)三种典型仲胺/叔胺组成MEA/PEG200非水吸收剂,系统研究了它们对CO 2捕集性能的调节作用。 。结果表明MEA/MDEA/PEG非水体系表现出优异的CO 2解吸性能。相应的最大再生效率达到82.1%,明显高于不添加MDEA的情况。第8次再生循环后仍保持较高的CO 2解吸效率(79%)。热力学分析表明,MEA/MDEA/PEG非水体系解吸反应热仅为1.40 GJ/吨CO 2,比非水MEA/PEG200吸收剂低20%。其再生总能耗为每吨CO 2 1.90GJ ,比MEA水溶液降低48.1%。FT-IR、13 C-NMR 和 DFT 计算表明,MDEA 调节剂的引入将导致两性离子和 MDEA 之间的反应能垒比两性离子和 MEA 之间的反应能垒低得多。此外,它会诱导质子化MDEA(MDEAH + )的形成,这更容易MDEA再生。
更新日期:2023-11-29
中文翻译:

一种基于 N-甲基二乙醇胺调节的新型二胺非水吸收剂,用于高效节能的二氧化碳捕获
非水吸收剂由于其较低的显热和蒸发潜热而受到越来越多的关注。但其低CO 2解吸效率和高解吸反应热仍然是实际应用的主要限制。在此,提出了一种基于烷醇胺调节的新型非水二胺吸收剂。实验中,采用单乙醇胺(MEA)作为主要吸收剂,以保证较高的CO 2吸收能力和吸收率,同时采用聚乙二醇200(PEG200)作为助溶剂,以降低蒸发潜热。随后,分别与N-甲基二乙醇胺(MDEA)、二乙醇胺(DEA)和三乙醇胺(TEA)三种典型仲胺/叔胺组成MEA/PEG200非水吸收剂,系统研究了它们对CO 2捕集性能的调节作用。 。结果表明MEA/MDEA/PEG非水体系表现出优异的CO 2解吸性能。相应的最大再生效率达到82.1%,明显高于不添加MDEA的情况。第8次再生循环后仍保持较高的CO 2解吸效率(79%)。热力学分析表明,MEA/MDEA/PEG非水体系解吸反应热仅为1.40 GJ/吨CO 2,比非水MEA/PEG200吸收剂低20%。其再生总能耗为每吨CO 2 1.90GJ ,比MEA水溶液降低48.1%。FT-IR、13 C-NMR 和 DFT 计算表明,MDEA 调节剂的引入将导致两性离子和 MDEA 之间的反应能垒比两性离子和 MEA 之间的反应能垒低得多。此外,它会诱导质子化MDEA(MDEAH + )的形成,这更容易MDEA再生。






































 京公网安备 11010802027423号
京公网安备 11010802027423号