当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereocontrolled Synthesis of α-3-Deoxy-d-manno-oct-2-ulosonic Acid (α-Kdo) Glycosides Using C3-p-Tolylthio-Substituted Kdo Donors: Access to Highly Branched Kdo Oligosaccharides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-11-28 , DOI: 10.1002/anie.202313985 Ao Sun 1 , Zipeng Li 1 , Yuchao Wang 1 , Shuai Meng 2 , Xiao Zhang 1 , Xiangbao Meng 1 , Shuchun Li 1 , Zhongtang Li 1 , Zhongjun Li 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-11-28 , DOI: 10.1002/anie.202313985 Ao Sun 1 , Zipeng Li 1 , Yuchao Wang 1 , Shuai Meng 2 , Xiao Zhang 1 , Xiangbao Meng 1 , Shuchun Li 1 , Zhongtang Li 1 , Zhongjun Li 1
Affiliation
|
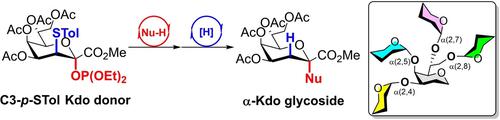
A modified donor has been developed for the glycosylation of 3-deoxy-d-manno-2-octulosonic acid (Kdo). The high reactivity and wide substrate scope of the donor enabled the synthesis of a range of Kdo-containing glycosides with complete α-stereoselectivity without the formation of 2,3-ene by-products. Several natural oligosaccharides were synthesized by a stepwise or one-pot process, including a highly branched Kdo pentasaccharide.
中文翻译:

使用 C3-对甲苯硫基取代的 Kdo 供体立体控制合成 α-3-脱氧-d-甘露-辛-2-酮糖酸 (α-Kdo) 糖苷:获得高度支化的 Kdo 寡糖
已开发出一种用于 3-脱氧-d-甘露-2-辛糖酸 (Kdo) 糖基化的修饰供体。供体的高反应活性和广泛的底物范围使得能够合成一系列具有完全α-立体选择性的含Kdo糖苷,而不形成2,3-烯副产物。通过分步或一锅法合成了几种天然低聚糖,包括高度支化的 Kdo 五糖。
更新日期:2023-11-28
中文翻译:

使用 C3-对甲苯硫基取代的 Kdo 供体立体控制合成 α-3-脱氧-d-甘露-辛-2-酮糖酸 (α-Kdo) 糖苷:获得高度支化的 Kdo 寡糖
已开发出一种用于 3-脱氧-d-甘露-2-辛糖酸 (Kdo) 糖基化的修饰供体。供体的高反应活性和广泛的底物范围使得能够合成一系列具有完全α-立体选择性的含Kdo糖苷,而不形成2,3-烯副产物。通过分步或一锅法合成了几种天然低聚糖,包括高度支化的 Kdo 五糖。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号