European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2023-11-26 , DOI: 10.1016/j.ejps.2023.106655
T Huzjak 1 , O Jakasanovski 2 , K Berginc 2 , V Puž 2 , K Zajc-Kreft 2 , Ž Jeraj 2 , B Janković 2
|
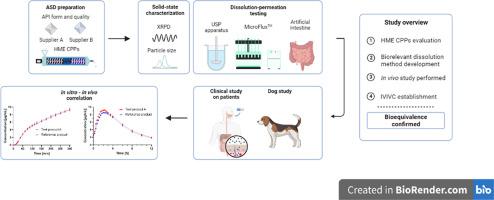
Hot-melt extrusion is often used to prepare amorphous solid dispersion to overcome low drug solubility and enhance bio-performance of the formulation. Due to the uniqueness of each drug – polymer combination and its physico-chemical properties, setting the appropriate HME barrel temperature, feed rate and screw speed ensures drug amorphization, absence of residual crystallinity, absence of water, and a suitable drug release profile. In this research, samples with BCS II/IV model drug and PVP/VA polymer were prepared to evaluate the impact of HME process parameters, incoming drug form (anhydrous vs. hydrate), and drug supplier (i.e., impurity profile), on biorelevant drug release. This study provides a relationship between observed in vitro supersaturation and precipitation behavior of amorphous solid dispersion formulation with in vivo results, on patients, by using the acceptor profile of side-by-side dissolution-permeation apparatus.
An in vitro dissolution method, in small volumes, in an apparatus with paddles and dissolution-permeation side-by-side method was developed on the MicroFlux™ apparatus to assess if the differences observed in vitro bears relevance to the bioequivalence outcome in vivo. The former was used to guide the generic drug product development due to high discriminatory strength, while the latter was biorelevant, due to the inclusion of the second compartment assuring absorptive environment to capture the impact of supersaturation and subsequent precipitation on bioavailability. Bio-relevancy of the in vitro method was confirmed with the in vivo dog study and clinical study on patients, and an in vitro – in vivo correlation was established.
For the investigated BCS II/IV drug, this research highlights the importance of considering supersaturation and formation of colloidal species during amorphous solid dispersion release testing to assure product quality, safety and efficacy.
中文翻译:

通过合理开发生物相关溶出渗透方法克服无定形固体分散体中的药物杂质挑战
热熔挤出常用于制备无定形固体分散体,以克服药物溶解度低的问题并增强制剂的生物性能。由于每种药物-聚合物组合及其物理化学性质的独特性,设置适当的 HME 料筒温度、进料速率和螺杆速度可确保药物非晶化、无残留结晶、无水和合适的药物释放曲线。在本研究中,制备了 BCS II/IV 模型药物和 PVP/VA 聚合物的样品,以评估 HME 工艺参数、传入药物形式(无水与水合物)和药物供应商(即杂质谱)对生物相关性的影响。药物释放。本研究通过使用并排溶解渗透装置的受体曲线,提供了观察到的无定形固体分散体制剂的体外过饱和度和沉淀行为与患者体内结果之间的关系。
在 MicroFlux™ 装置上开发了一种在带桨的装置中进行小体积体外溶出方法和溶出-渗透并行方法,以评估体外观察到的差异是否与体内生物等效性结果相关。前者由于具有高辨别力而被用来指导仿制药产品的开发,而后者则具有生物相关性,因为包含第二个隔室可确保吸收环境以捕获过饱和和随后的沉淀对生物利用度的影响。体外方法的生物相关性通过狗体内研究和患者临床研究得到证实,并建立了体外-体内相关性。
对于所研究的 BCS II/IV 药物,本研究强调了在无定形固体分散体释放测试过程中考虑过饱和和胶体物质形成的重要性,以确保产品质量、安全性和有效性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号