European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-11-25 , DOI: 10.1016/j.ejmech.2023.115977 Xiuqi Wang 1 , Rosa Anna DeFilippis 2 , Tsigereda Weldemichael 3 , Naresh Gunaganti 3 , Phuc Tran 3 , Yuet-Kin Leung 4 , Neil P Shah 2 , Hong-Yu Li 3
|
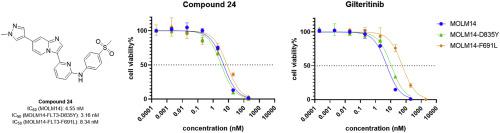
FLT3 activating mutations are detected in approximately 30 % of newly diagnosed acute myeloid leukemia (AML) cases, most commonly consisting of internal tandem duplication (ITD) mutations in the juxtamembrane region. Recently, several FLT3 inhibitors have demonstrated clinical activity and three are currently approved – midostaurin, quizartinib, and gilteritinib. Midostaurin is a first-generation FLT3 inhibitor with minimal activity as monotherapy. Midostaurin lacks selectivity and is only approved by the USFDA for use in combination with other chemotherapy agents. The second-generation inhibitors quizartinib and gilteritinib display improved specificity and selectivity, and have been approved for use as monotherapy. However, their clinical efficacies are limited in part due to the emergence of drug-resistant FLT3 secondary mutations in the tyrosine kinase domain at positions D835 and F691. Therefore, in order to overcome drug resistance and further improve outcomes, new compounds targeting FLT3-ITD with secondary mutants are urgently needed. In this study, through the structural modification of a reported compound Ling-5e, we identified compound 24 as a FLT3 inhibitor that is equally potent against FLT3-ITD and the clinically relevant mutants FLT3-ITD/D835Y, and FLT3-ITD/F691L. Its inhibitory effects were demonstrated in both cell viability assays and western blots analyses. When tested against cell lines lacking activating mutations in FLT3, no non-specific cytotoxicity effect was observed. Interestingly, molecular docking results showed that compound 24 may adopt different binding conformations with FLT3-F691L compared to FLT3, which may explain its retained activity against FLT3-ITD/F691L. In summary, compound 24 has inhibition potency on FLT3 comparable to gilteritinib, but a more balanced inhibition on FLT3 secondary mutations, especially FLT3-ITD/F691L which is gilteritinib resistant. Compound 24 may serve as a promising lead for the drug development of either primary or relapsed AML with FLT3 secondary mutations.
中文翻译:

咪唑并[1,2-a]吡啶-吡啶衍生物有效抑制 FLT3-ITD 和 FLT3-ITD 二次突变体,包括 gilteritinib 耐药性 FLT3-ITD/F691L
大约 30% 的新诊断急性髓系白血病 (AML) 病例中检测到 FLT3 激活突变,最常见的是近膜区域的内部串联重复 (ITD) 突变。最近,几种FLT3抑制剂已表现出临床活性,其中三种抑制剂目前已获得批准——米多妥林、quizartinib和gilteritinib。 Midostaurin 是第一代 FLT3 抑制剂,作为单一疗法时活性极小。 Midostaurin缺乏选择性,仅被美国FDA批准与其他化疗药物联合使用。第二代抑制剂 quizartinib 和 gilteritinib 显示出更高的特异性和选择性,并已被批准用作单一疗法。然而,其临床疗效在一定程度上受到限制,因为酪氨酸激酶结构域 D835 和 F691 位置出现了耐药性 FLT3 二次突变。因此,为了克服耐药性并进一步改善结果,迫切需要具有二次突变体的靶向FLT3-ITD的新化合物。在这项研究中,通过对已报道的化合物Ling-5e进行结构修饰,我们鉴定出化合物24是一种 FLT3 抑制剂,对 FLT3-ITD 和临床相关突变体 FLT3-ITD/D835Y 和 FLT3-ITD/F691L 具有同等效力。其抑制作用在细胞活力测定和蛋白质印迹分析中得到证实。当针对缺乏 FLT3 激活突变的细胞系进行测试时,没有观察到非特异性细胞毒性作用。 有趣的是,分子对接结果表明,与FLT3相比,化合物24与FLT3-F691L可能采取不同的结合构象,这可能解释了其保留的针对FLT3-ITD/F691L的活性。综上所述,化合物24对FLT3的抑制效力与gilteritinib相当,但对FLT3二次突变的抑制更为平衡,尤其是对gilteritinib耐药的FLT3-ITD/F691L。化合物24可能作为具有 FLT3 二次突变的原发性或复发性 AML 药物开发的有前途的先导药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号