European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-11-22 , DOI: 10.1016/j.ejmech.2023.115984 Guofeng Chen 1 , Hang Xie 2 , Mengyuan You 2 , Jiayuan Liu 3 , Qiang Shao 4 , Minjun Li 5 , Haixia Su 4 , Yechun Xu 6
|
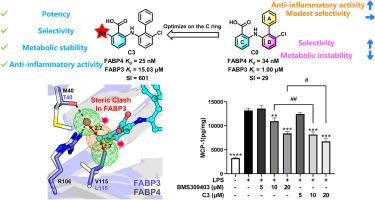
Fatty-acid binding protein 4 (FABP4) presents an attractive target for therapeutic intervention in metabolic and inflammatory diseases in recent years. However, highly similar three-dimensional structures and fatty acid binding ability of multiple FABP family members pose a significant challenge in design of FABP4-selective inhibitors. Particularly, inhibition of FABP3 raises safety concerns such as cardiac dysfunction and exercise intolerance. Here, we reported the discovery of new FABP4 inhibitors with high selectivity over FABP3 by exploiting the little structural difference in the ligand binding pockets of FABP4 and FABP3. On the basis of our previously reported FABP4 inhibitors with nanomolar potency, different substituents were further introduced to perfectly occupy two sub-pockets of FABP4 that are distinct from those of FABP3. Remarkably, a single methyl group introduction leads to the discovery of compound C3 that impressively exhibits a 601-fold selectivity over FABP3 when maintained nanomolar binding affinity for FABP4. Moreover, C3 also shows good metabolic stability and potent cellular anti-inflammatory activity, making it a promising inhibitor for further development. Therefore, the present study highlights the utility of the structure-based rational design strategy for seeking highly selective and potent inhibitors of FABP4 and the importance of identifying the appropriate subsite as well as substituent for gaining the desired selectivity.
中文翻译:

基于结构的设计对 FABP3 具有高选择性的强效 FABP4 抑制剂
近年来,脂肪酸结合蛋白 4 (FABP4) 成为代谢和炎症疾病治疗干预的一个有吸引力的靶点。然而,多个FABP家族成员高度相似的三维结构和脂肪酸结合能力对FABP4选择性抑制剂的设计提出了重大挑战。特别是,抑制 FABP3 会引发安全问题,例如心脏功能障碍和运动不耐受。在这里,我们报道了通过利用 FABP4 和 FABP3 配体结合口袋的微小结构差异,发现了比 FABP3 具有高选择性的新型 FABP4 抑制剂。在我们之前报道的具有纳摩尔效力的FABP4抑制剂的基础上,进一步引入不同的取代基以完美地占据FABP4的两个与FABP3不同的子口袋。值得注意的是,单甲基基团的引入导致了化合物C3的发现,当保持对 FABP4 的纳摩尔结合亲和力时,其选择性比 FABP3 显着提高 601 倍。此外, C3还表现出良好的代谢稳定性和有效的细胞抗炎活性,使其成为有前途的进一步开发的抑制剂。因此,本研究强调了基于结构的合理设计策略在寻找高选择性和有效的 FABP4 抑制剂方面的实用性,以及确定适当的亚位点和取代基以获得所需选择性的重要性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号