当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of Pain Perception by Microbiota in Parkinson Disease
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2024-01-01 , DOI: 10.1124/pharmrev.122.000674 Zulmary Manjarres 1 , Margarita Calvo 2 , Rodrigo Pacheco 2
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2024-01-01 , DOI: 10.1124/pharmrev.122.000674 Zulmary Manjarres 1 , Margarita Calvo 2 , Rodrigo Pacheco 2
Affiliation
|
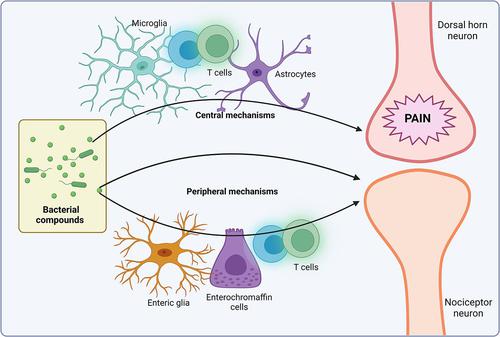
Pain perception involves current stimulation in peripheral nociceptive nerves and the subsequent stimulation of postsynaptic excitatory neurons in the spinal cord. Importantly, in chronic pain, the neural activity of both peripheral nociceptors and postsynaptic neurons in the central nervous system is influenced by several inflammatory mediators produced by the immune system. Growing evidence has indicated that the commensal microbiota plays an active role in regulating pain perception by either acting directly on nociceptors or indirectly through the modulation of the inflammatory activity on immune cells. This symbiotic relationship is mediated by soluble bacterial mediators or intrinsic structural components of bacteria that act on eukaryotic cells, including neurons, microglia, astrocytes, macrophages, T cells, enterochromaffin cells, and enteric glial cells. The molecular mechanisms involve bacterial molecules that act directly on neurons, affecting their excitability, or indirectly on non-neuronal cells, inducing changes in the production of proinflammatory or anti-inflammatory mediators. Importantly, Parkinson disease, a neurodegenerative and inflammatory disorder that affects mainly the dopaminergic neurons implicated in the control of voluntary movements, involves not only a motor decline but also nonmotor symptomatology, including chronic pain. Of note, several recent studies have shown that Parkinson disease involves a dysbiosis in the composition of the gut microbiota. In this review, we first summarize, integrate, and classify the molecular mechanisms implicated in the microbiota-mediated regulation of chronic pain. Second, we analyze the changes on the commensal microbiota associated to Parkinson disease and propose how these changes affect the development of chronic pain in this pathology.
中文翻译:

帕金森病中微生物群对疼痛感知的调节
疼痛感知涉及外周伤害性神经的电流刺激以及随后对脊髓中突触后兴奋性神经元的刺激。重要的是,在慢性疼痛中,中枢神经系统中的外周伤害感受器和突触后神经元的神经活动受到免疫系统产生的几种炎症介质的影响。越来越多的证据表明,共生微生物群通过直接作用于伤害感受器或通过调节免疫细胞的炎症活动间接作用,在调节疼痛感知方面发挥着积极作用。这种共生关系是由作用于真核细胞(包括神经元、小胶质细胞、星形胶质细胞、巨噬细胞、T 细胞、肠嗜铬细胞和肠神经胶质细胞)的可溶性细菌介质或细菌的内在结构成分介导的。分子机制涉及细菌分子直接作用于神经元,影响其兴奋性,或间接作用于非神经元细胞,诱导促炎或抗炎介质产生的变化。重要的是,帕金森病是一种神经退行性和炎症性疾病,主要影响与随意运动控制有关的多巴胺能神经元,不仅涉及运动衰退,还涉及非运动症状,包括慢性疼痛。值得注意的是,最近的几项研究表明,帕金森病涉及肠道微生物群组成的失调。在这篇综述中,我们首先总结、整合和分类了微生物介导的慢性疼痛调节所涉及的分子机制。 其次,我们分析了与帕金森病相关的共生微生物群的变化,并提出了这些变化如何影响这种病理学中慢性疼痛的发展。
更新日期:2023-12-16
中文翻译:

帕金森病中微生物群对疼痛感知的调节
疼痛感知涉及外周伤害性神经的电流刺激以及随后对脊髓中突触后兴奋性神经元的刺激。重要的是,在慢性疼痛中,中枢神经系统中的外周伤害感受器和突触后神经元的神经活动受到免疫系统产生的几种炎症介质的影响。越来越多的证据表明,共生微生物群通过直接作用于伤害感受器或通过调节免疫细胞的炎症活动间接作用,在调节疼痛感知方面发挥着积极作用。这种共生关系是由作用于真核细胞(包括神经元、小胶质细胞、星形胶质细胞、巨噬细胞、T 细胞、肠嗜铬细胞和肠神经胶质细胞)的可溶性细菌介质或细菌的内在结构成分介导的。分子机制涉及细菌分子直接作用于神经元,影响其兴奋性,或间接作用于非神经元细胞,诱导促炎或抗炎介质产生的变化。重要的是,帕金森病是一种神经退行性和炎症性疾病,主要影响与随意运动控制有关的多巴胺能神经元,不仅涉及运动衰退,还涉及非运动症状,包括慢性疼痛。值得注意的是,最近的几项研究表明,帕金森病涉及肠道微生物群组成的失调。在这篇综述中,我们首先总结、整合和分类了微生物介导的慢性疼痛调节所涉及的分子机制。 其次,我们分析了与帕金森病相关的共生微生物群的变化,并提出了这些变化如何影响这种病理学中慢性疼痛的发展。






























 京公网安备 11010802027423号
京公网安备 11010802027423号