当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Enantioselective Chiral Diol-Catalyzed Conjugate Addition of Boronic Acids to α,β-Unsaturated Trifluoromethyl Ketones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-11-25 , DOI: 10.1021/acs.joc.3c02281 Ya-Jing Hou 1 , Lu Zhao 1 , Guo-Li Chai 1 , Kangbao Zhong 1 , Junbiao Chang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-11-25 , DOI: 10.1021/acs.joc.3c02281 Ya-Jing Hou 1 , Lu Zhao 1 , Guo-Li Chai 1 , Kangbao Zhong 1 , Junbiao Chang 1
Affiliation
|
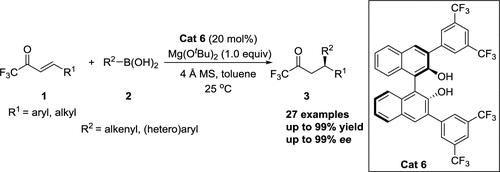
The (R)-3,3′-(3,5-(CF3)2-C6H3)2-BINOL-catalyzed enantioselective conjugate addition of organic boronic acids to α,β-unsaturated 1,1,1-trifluoromethyl ketones affords corresponding addition products bearing a stereogenic center at the β-position in moderate to high yields and excellent enantioselectivities (up to 99% ee), without any 1,2-addition product formation. Moreover, this catalytic protocol features mild reaction conditions, a broad substrate scope, suitability for alkenylboronic acids and (hetero)arylboronic acids, and easy scale-up.
中文翻译:

高对映选择性手性二醇催化硼酸与 α,β-不饱和三氟甲基酮的共轭加成
( R )-3,3'-(3,5-(CF 3 ) 2 -C 6 H 3 ) 2 -BINOL 催化有机硼酸与 α,β-不饱和 1,1,1- 的对映选择性共轭加成三氟甲基酮以中等到高产率提供在 β 位带有立体中心的相应加成产物,并具有优异的对映选择性(高达 99% ee),且不形成任何 1,2-加成产物。此外,该催化方案具有反应条件温和、底物范围广、适用于烯基硼酸和(杂)芳基硼酸且易于放大的特点。
更新日期:2023-11-25
中文翻译:

高对映选择性手性二醇催化硼酸与 α,β-不饱和三氟甲基酮的共轭加成
( R )-3,3'-(3,5-(CF 3 ) 2 -C 6 H 3 ) 2 -BINOL 催化有机硼酸与 α,β-不饱和 1,1,1- 的对映选择性共轭加成三氟甲基酮以中等到高产率提供在 β 位带有立体中心的相应加成产物,并具有优异的对映选择性(高达 99% ee),且不形成任何 1,2-加成产物。此外,该催化方案具有反应条件温和、底物范围广、适用于烯基硼酸和(杂)芳基硼酸且易于放大的特点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号