Redox Biology ( IF 10.7 ) Pub Date : 2023-11-19 , DOI: 10.1016/j.redox.2023.102965 Yang Luo 1 , Laurent Chatre 2 , Shaden Melhem 3 , Zayana M Al-Dahmani 4 , Natalie Z M Homer 5 , Anneke Miedema 6 , Leo E Deelman 7 , Matthew R Groves 4 , Martin Feelisch 8 , Nicholas M Morton 9 , Amalia Dolga 10 , Harry van Goor 6
|
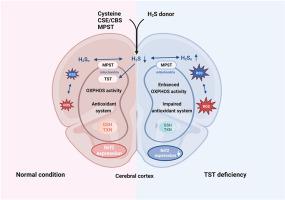
Thiosulfate sulfurtransferase (TST, EC 2.8.1.1) was discovered as an enzyme that detoxifies cyanide by conversion to thiocyanate (rhodanide) using thiosulfate as substrate; this rhodanese activity was subsequently identified to be almost exclusively located in mitochondria. More recently, the emphasis regarding its function has shifted to hydrogen sulfide metabolism, antioxidant defense, and mitochondrial function in the context of protective biological processes against oxidative distress. While TST has been described to play an important role in liver and colon, its function in the brain remains obscure. In the present study, we therefore sought to address its potential involvement in maintaining cerebral redox balance in a murine model of global TST deficiency (Tst−/− mice), primarily focusing on characterizing the biochemical phenotype of TST loss in relation to neuronal activity and sensitivity to oxidative stress under basal conditions. Here, we show that TST deficiency is associated with a perturbation of the reactive species interactome in the brain cortex secondary to altered ROS and RSS (specifically, polysulfide) generation as well as mitochondrial OXPHOS remodeling. These changes were accompanied by aberrant Nrf2-Keap1 expression and thiol-dependent antioxidant function. Upon challenging mice with the redox-active herbicide paraquat (25 mg/kg i.p. for 24 h), Tst−/− mice displayed a lower antioxidant capacity compared to wildtype controls (C57BL/6J mice). These results provide a first glimpse into the molecular and metabolic changes of TST deficiency in the brain and suggest that pathophysiological conditions associated with aberrant TST expression and/or activity renders neurons more susceptible to oxidative stress-related malfunction.
中文翻译:

硫代硫酸盐硫转移酶缺乏会促进大脑氧化应激和 NRF2 功能异常
硫代硫酸盐硫转移酶(TST,EC 2.8.1.1)被发现是一种通过使用硫代硫酸盐作为底物转化为硫氰酸盐(硫氰酸)来解毒氰化物的酶;随后发现这种硫氰酸酶活性几乎完全位于线粒体中。最近,在针对氧化应激的保护性生物过程的背景下,对其功能的重点已转向硫化氢代谢、抗氧化防御和线粒体功能。虽然 TST 被认为在肝脏和结肠中发挥着重要作用,但它在大脑中的功能仍然不清楚。因此,在本研究中,我们试图解决其在整体 TST 缺乏的小鼠模型( Tst −/−小鼠)中维持大脑氧化还原平衡的潜在参与,主要侧重于表征 TST 损失与神经元活动相关的生化表型和基础条件下对氧化应激的敏感性。在这里,我们发现 TST 缺乏与大脑皮层中活性物质相互作用组的扰动有关,继发于 ROS 和 RSS(特别是多硫化物)生成的改变以及线粒体 OXPHOS 重塑。这些变化伴随着异常的 Nrf2-Keap1 表达和硫醇依赖性抗氧化功能。用氧化还原活性除草剂百草枯(25 mg/kg ip,持续 24 小时)攻击小鼠后,与野生型对照(C57BL/6J 小鼠)相比, Tst −/−小鼠表现出较低的抗氧化能力。 这些结果使人们首次了解大脑中 TST 缺乏的分子和代谢变化,并表明与异常 TST 表达和/或活性相关的病理生理条件使神经元更容易受到氧化应激相关功能障碍的影响。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号