Journal of Energy Storage ( IF 8.9 ) Pub Date : 2023-11-21 , DOI: 10.1016/j.est.2023.109640 Zahra Tabandeh , Farideh Zergani , Somayeh Ghasemi
|
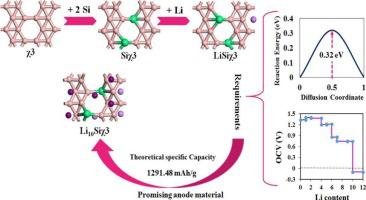
A favorable anodic candidate with significant potential is currently in high demand for Lithium-ion batteries. The χ3 borophene, a two-dimensional material, shows potential as an anode in Li-ion batteries. However, its original structure requires improvement to enhance its Li diffusivity and specific capacity. This research investigates the modification of χ3 borophene by silicon atoms, resulting in a new structure called siliborophene, using density functional theory. Ab-initio molecular dynamics simulations confirm the stability of siliborophene structure at room temperature. The Siχ3 structure hosts adsorption sites for the Li atom, and after exploring various positions, the most favorable configuration exhibits an adsorption energy of −1.33 eV. The siliborophene surface can adsorbed up to 13 Li atoms but according to the further analyses the results of open-circuit voltage suggest that the siliborophene structure can store 10 Li atoms, so Li10Siχ3 structure is considered as the structure with maximum adsorption capacity. The interaction between the surface and Li atoms is stronger in this configuration, with −1.10 eV adsorption energy which yields a maximum specific capacity of 1291 mAh/g. Analysis of the partial density of states and band structure reveals metallic characteristics of the surface during lithiation. Furthermore, using the CI-NEB approach, the barrier activation energy for Li diffusion was calculated, showing exceptionally low values at 0.32 eV, enabling rapid Li diffusion on the surface. These findings indicate that siliborophene holds promise as a potential anode material for lithium-ion batteries.
中文翻译:

揭示硅硼吩作为先进锂离子电池阳极材料的潜力:DFT 和从头算分子动力学
目前,锂离子电池对一种具有巨大潜力的有利阳极候选材料的需求量很大。χ3 硼烯是一种二维材料,具有作为锂离子电池阳极的潜力。然而,其原始结构需要改进以提高其锂扩散率和比容量。这项研究利用密度泛函理论研究了硅原子对 χ3 硼吩的修饰,产生了一种称为硅硼吩的新结构。从头算分子动力学模拟证实了硅硼吩结构在室温下的稳定性。Siχ3结构具有Li原子的吸附位点,在探索了各种位置后,最有利的构型表现出-1.33 eV的吸附能。硅硼烯表面最多可吸附13个Li原子,但根据开路电压的进一步分析结果表明,硅硼烯结构可存储10个Li原子,因此Li 10 Siχ3结构被认为是吸附容量最大的结构。在这种结构中,表面与锂原子之间的相互作用更强,吸附能为-1.10 eV,最大比容量为1291 mAh/g。对部分态密度和能带结构的分析揭示了锂化过程中表面的金属特征。此外,使用 CI-NEB 方法计算了 Li 扩散的势垒活化能,显示出极低的值,为 0.32 eV,使得 Li 在表面快速扩散。这些发现表明,硅硼吩有望成为锂离子电池的潜在阳极材料。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号