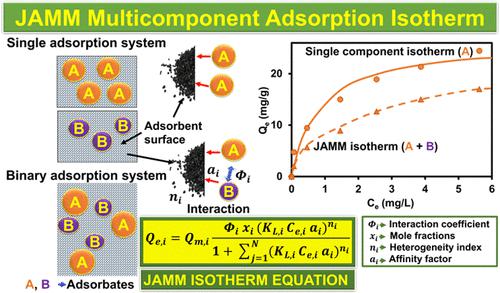
在过去的几十年里,研究人员通过开发各种简单的吸附等温线模型,为了解吸附做出了巨大的努力。然而,尽管许多污染物在自然界中通常以多组分混合物的形式存在,但多组分吸附等温线受到的关注有限,并且仍然是一个研究不足的领域。我们在这里提出了一种新的多组分吸附等温线模型,名为 Jeppu Amrutha Manipal 多组分 (JAMM) 等温线,可以缓解这个问题。我们首先使用活性炭上砷和氟化物竞争吸附的实验数据集开发了 JAMM 多组分等温线。然后,我们测试了 JAMM 多组分等温线,以进行镉和锌竞争吸附的案例研究。接下来,我们使用铜和铬的另一个竞争吸附案例研究进一步评估了 JAMM 等温线。通过广泛的验证研究和误差分析,JAMM 等温线能够证明其在准确预测多个多组分吸附系统中的吸附行为方面的功效。与其他多组分等温线相比,JAMM 等温线的主要优点是它利用并利用单组分吸附参数来模拟多组分等温线。所提出的 JAMM 分析等温线模型还结合了组分之间的相互作用、摩尔分数参数和异质性指数,为多组分吸附提供了更全面的建模框架。引入摩尔分数项来描述基于每种组分分子的相对数量的吸附位点的分布。引入了交互系数的附加术语来表示交互作用。 在对 JAMM 进行验证时,通过三个实验案例研究(可忽略、小型且高度竞争的吸附物系统),显示出令人印象深刻的预测,平均归一化绝对百分比误差为 6.05%,平均R 2为 0.86,凸显了模型的稳健性和多功能性和可靠性。我们认为,新的 JAMM 等温线建模框架可能会为分析和预测多组分吸附系统提供有效的工具,从而对化学工程、环境工程和材料科学应用产生深远的帮助。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Development of a Multicomponent Adsorption Isotherm Equation and Its Validation by Modeling
Researchers have made significant efforts over the past few decades to understand adsorption by developing various simple adsorption isotherm models. However, though many contaminants usually occur as multicomponent mixtures in nature, multicomponent adsorption isotherms have received limited attention and remain an area of inadequate research. We have presented here in a new multicomponent adsorption isotherm model, named the Jeppu Amrutha Manipal Multicomponent (JAMM) isotherm, that can alleviate this problem. We first developed the JAMM multicomponent isotherm using our experimental data sets of arsenic and fluoride competitive adsorption on activated carbon. We then tested the JAMM multicomponent isotherm for a case study of cadmium and zinc competitive adsorption. Next, we further assessed the JAMM isotherm using another competitive adsorption case study of copper and chromium. Through extensive validation studies and error analysis, the JAMM isotherm was able to demonstrate its efficacy in predicting the adsorption behavior in several multicomponent adsorption systems accurately. The main advantage of JAMM isotherm over other multicomponent isotherms is that it utilizes and leverages the single-component adsorption parameters to simulate multicomponent isotherms. The proposed JAMM analytical isotherm model furthermore incorporates the interaction between the components, a mole fraction parameter, and a heterogeneity index, providing a more comprehensive modeling framework for multicomponent adsorption. The mole fraction term was introduced for the distribution of adsorption sites based on the relative number of molecules of each component. An additional term for interaction coefficient was introduced for the representation of interactions. During the validation of JAMM with three experimental case studies with negligible, small, and high competition systems of adsorbates, impressive predictions were exhibited, with the average normalized absolute percentage error as 6.05% and average R2 as 0.86, highlighting the model’s robustness, versatility, and reliability. We propose that the new JAMM isotherm modeling framework might profoundly help in chemical engineering, environmental engineering, and materials science applications by providing a potent tool for analyzing and predicting multicomponent adsorption systems.


































 京公网安备 11010802027423号
京公网安备 11010802027423号