当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A telluroviologen-anchored tetraphenylporphyrin as sonosensitizer for periodontitis sonodynamic therapy
Biomaterials ( IF 12.8 ) Pub Date : 2023-11-23 , DOI: 10.1016/j.biomaterials.2023.122407 Qi Sun 1 , Weijie Song 1 , Yujing Gao 1 , Rui Ding 2 , Shuai Shi 1 , Suxia Han 3 , Guoping Li 1 , Dandan Pei 4 , Ang Li 4 , Gang He 2
Biomaterials ( IF 12.8 ) Pub Date : 2023-11-23 , DOI: 10.1016/j.biomaterials.2023.122407 Qi Sun 1 , Weijie Song 1 , Yujing Gao 1 , Rui Ding 2 , Shuai Shi 1 , Suxia Han 3 , Guoping Li 1 , Dandan Pei 4 , Ang Li 4 , Gang He 2
Affiliation
|
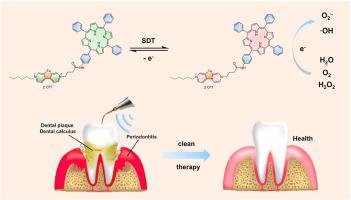
Periodontitis is a chronic disease caused by bacteria (e.g. Porphyromonas gingivalis, P.gingivalis ) that currently lacks effective non-invasive treatment options. Sonodynamic therapy (SDT) is an emerging non-invasive antimicrobial therapeutic strategy. Since ultrasonic tooth cleaning is widely used in dental treatments, SDT has significant potential for the facile implementation of treat periodontitis. However, hypoxia in periodontitis severely limits the effectiveness of traditional sonosensitizers. To address this issue, we have developed a new sonosensitizer termed as TPP-TeV, which combines the traditional sonosensitizer tetraphenylporphyrin (TPP) with a new photosensitizer telluroviologen (TeV). Under ultrasound radiation, TPP-TeV can produce numerous cationic free radicals (TPP-TeV•), which subsequently generate ROS free radicals (O2 •− , •OH) efficiently via electron transfer mechanism, resulting in the effective killing of anaerobic P.gingivalis both in vivo and in vitro. As a result, the dental environment is improved, and the inhibition rate of alveolar bone loss reaches 80 %. The introduction of tellurium into the viologen molecule induces changes in its reduction potential, resulting in increased rigidity of the molecule. This modification systematically reduces the biotoxicity of our novel sonosensitizer by 75 % at 50 μM based on bacterial experiments. These promising findings could potentially establish new options for sonodynamic therapy (SDT) in periodontitis clinical treatments.
中文翻译:

碲紫精锚定的四苯基卟啉作为牙周炎声动力治疗的声敏剂
牙周炎是一种由细菌(例如牙龈卟啉单胞菌、牙龈卟啉单胞菌)引起的慢性疾病,目前缺乏有效的非侵入性治疗方案。声动力疗法(SDT)是一种新兴的非侵入性抗菌治疗策略。由于超声波洁牙在牙科治疗中广泛应用,SDT 在轻松实施牙周炎治疗方面具有巨大潜力。然而,牙周炎中的缺氧严重限制了传统声敏剂的有效性。为了解决这个问题,我们开发了一种名为TPP-TeV的新型声敏剂,它将传统的声敏剂四苯基卟啉(TPP)与新型光敏剂碲紫罗碱(TeV)结合起来。在超声辐射下,TPP-TeV可产生大量的阳离子自由基(TPP-TeV•),随后通过电子转移机制高效产生ROS自由基(O2•−、•OH),从而有效杀灭厌氧牙龈卟啉单胞菌体内和体外。从而改善了牙齿环境,牙槽骨丢失的抑制率达到80%。将碲引入紫精分子中会引起其还原电位的变化,从而导致分子的刚性增加。根据细菌实验,这种修饰系统地将我们的新型声敏剂在 50 μM 时的生物毒性降低了 75%。这些有希望的发现可能会为牙周炎临床治疗中的声动力疗法(SDT)建立新的选择。
更新日期:2023-11-23
中文翻译:

碲紫精锚定的四苯基卟啉作为牙周炎声动力治疗的声敏剂
牙周炎是一种由细菌(例如牙龈卟啉单胞菌、牙龈卟啉单胞菌)引起的慢性疾病,目前缺乏有效的非侵入性治疗方案。声动力疗法(SDT)是一种新兴的非侵入性抗菌治疗策略。由于超声波洁牙在牙科治疗中广泛应用,SDT 在轻松实施牙周炎治疗方面具有巨大潜力。然而,牙周炎中的缺氧严重限制了传统声敏剂的有效性。为了解决这个问题,我们开发了一种名为TPP-TeV的新型声敏剂,它将传统的声敏剂四苯基卟啉(TPP)与新型光敏剂碲紫罗碱(TeV)结合起来。在超声辐射下,TPP-TeV可产生大量的阳离子自由基(TPP-TeV•),随后通过电子转移机制高效产生ROS自由基(O2•−、•OH),从而有效杀灭厌氧牙龈卟啉单胞菌体内和体外。从而改善了牙齿环境,牙槽骨丢失的抑制率达到80%。将碲引入紫精分子中会引起其还原电位的变化,从而导致分子的刚性增加。根据细菌实验,这种修饰系统地将我们的新型声敏剂在 50 μM 时的生物毒性降低了 75%。这些有希望的发现可能会为牙周炎临床治疗中的声动力疗法(SDT)建立新的选择。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号