当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In-situ reconstructed Cu/Cu2O heterogeneous nanorods with oxygen vacancies for enhanced electrocatalytic nitrate reduction to ammonia
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-11-20 , DOI: 10.1016/j.cej.2023.147574 Yan Shi , Yumeng Li , Rujin Li , Xiaogang Zhao , Yanling Yu , Min Yang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-11-20 , DOI: 10.1016/j.cej.2023.147574 Yan Shi , Yumeng Li , Rujin Li , Xiaogang Zhao , Yanling Yu , Min Yang
|
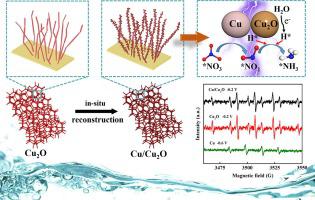
Electrocatalytic nitrate reduction to ammonia (ENRA) is crucial for environmental pollution treatment and sustainable energy development, but the complicated reduction mechanism of ENRA leads to limited ammonia selectivity and low Faradaic efficiency. In this work, Cu/Cu2 O heterogeneous nanorods with oxygen vacancies were successfully obtained by creating Cu sites via the in-situ reconstruction of Cu2 O nanorods. These oxygen vacancies can weaken the N-O bond. The Cu sites and heterogeneous interfaces were investigated using X-ray photoelectron spectroscopy Auger spectra and transmission electron microscopy. The active species trapping experiments and density functional theory (DFT) calculations demonstrated that the Cu sites promoted the reduction of NO 3 - NO 2 - NO 3 - 2 O facilitated the formation of a *NOH intermediate. In addition, abundant heterogeneous interfaces facilitated the reduction of N-containing intermediates by atomic hydrogen, leading to a high Faradaic efficiency of 84.93 % and ammonia yield of 4.58 mg/h/cm2 in neutral solution. Our findings will diversify the methodologies for constructing Cu/Cu2 O electrocatalysts and provide new insight into the mechanism of ammonia synthesis from nitrate reduction.
中文翻译:

原位重构具有氧空位的 Cu/Cu2O 异质纳米棒用于增强电催化硝酸盐还原成氨
电催化硝酸盐还原氨(ENRA)对于环境污染治理和可持续能源发展至关重要,但ENRA复杂的还原机理导致氨选择性有限且法拉第效率低。在这项工作中,通过原位重构Cu2O纳米棒创建Cu位点,成功获得了具有氧空位的Cu/Cu2O异质纳米棒。这些氧空位会削弱NO键。使用 X 射线光电子能谱、俄歇能谱和透射电子显微镜研究了铜位点和异质界面。活性物种捕获实验和密度泛函理论(DFT)计算表明,Cu位点对NO3-的强烈吸附促进了NO3-还原为NO2-,并且来自Cu2O的足够的原子氢促进了*的形成。 NOH中间体。此外,丰富的异质界面有利于原子氢还原含氮中间体,在中性溶液中法拉第效率高达84.93%,氨产率为4.58 mg/h/cm2。我们的研究结果将使构建 Cu/Cu2O 电催化剂的方法多样化,并为硝酸盐还原合成氨的机制提供新的见解。
更新日期:2023-11-20
中文翻译:

原位重构具有氧空位的 Cu/Cu2O 异质纳米棒用于增强电催化硝酸盐还原成氨
电催化硝酸盐还原氨(ENRA)对于环境污染治理和可持续能源发展至关重要,但ENRA复杂的还原机理导致氨选择性有限且法拉第效率低。在这项工作中,通过原位重构Cu2O纳米棒创建Cu位点,成功获得了具有氧空位的Cu/Cu2O异质纳米棒。这些氧空位会削弱NO键。使用 X 射线光电子能谱、俄歇能谱和透射电子显微镜研究了铜位点和异质界面。活性物种捕获实验和密度泛函理论(DFT)计算表明,Cu位点对NO3-的强烈吸附促进了NO3-还原为NO2-,并且来自Cu2O的足够的原子氢促进了*的形成。 NOH中间体。此外,丰富的异质界面有利于原子氢还原含氮中间体,在中性溶液中法拉第效率高达84.93%,氨产率为4.58 mg/h/cm2。我们的研究结果将使构建 Cu/Cu2O 电催化剂的方法多样化,并为硝酸盐还原合成氨的机制提供新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号