当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous and Selective Peptide Elongation in Water Driven by Aminoacyl Phosphate Esters and Phase Changes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-22 , DOI: 10.1021/jacs.3c07918 Kun Dai 1 , Mahesh D Pol 1 , Lenard Saile 1 , Arti Sharma 2 , Bin Liu 3 , Ralf Thomann 2, 4 , Johanna L Trefs 1, 5 , Danye Qiu 6 , Sandra Moser 6 , Stefan Wiesler 6 , Bizan N Balzer 1, 4, 5 , Thorsten Hugel 1, 5 , Henning J Jessen 1, 6 , Charalampos G Pappas 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-11-22 , DOI: 10.1021/jacs.3c07918 Kun Dai 1 , Mahesh D Pol 1 , Lenard Saile 1 , Arti Sharma 2 , Bin Liu 3 , Ralf Thomann 2, 4 , Johanna L Trefs 1, 5 , Danye Qiu 6 , Sandra Moser 6 , Stefan Wiesler 6 , Bizan N Balzer 1, 4, 5 , Thorsten Hugel 1, 5 , Henning J Jessen 1, 6 , Charalampos G Pappas 1
Affiliation
|
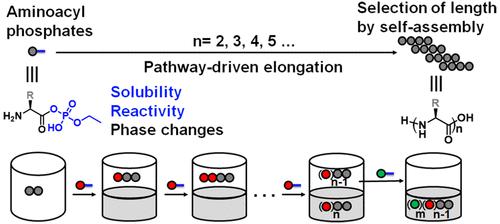
Nature chose phosphates to activate amino acids, where reactive intermediates and complex machinery drive the construction of polyamides. Outside of biology, the pathways and mechanisms that allow spontaneous and selective peptide elongation in aqueous abiotic systems remain unclear. Herein we work to uncover those pathways by following the systems chemistry of aminoacyl phosphate esters, synthetic counterparts of aminoacyl adenylates. The phosphate esters act as solubility tags, making hydrophobic amino acids and their oligomers soluble in water and enabling selective elongation and different pathways to emerge. Thus, oligomers up to dodecamers were synthesized in one flask and on the minute time scale, where consecutive additions activated autonomous phase changes. Depending on the pathway, the resulting phases initially carry nonpolar peptides and amphiphilic oligomers containing phosphate esters. During elongation and phosphate release, shorter oligomers dominate in solution, while the aggregated phase favors the presence of longer oligomers due to their self-assembly propensity. Furthermore we demonstrated that the solution phases can be isolated and act as a new environment for continuous elongation, by adding various phosphate esters. These findings suggest that the systems chemistry of aminoacyl phosphate esters can activate a selection mechanism for peptide bond formation by merging aqueous synthesis and self-assembly.
中文翻译:

氨酰磷酸酯和相变驱动的水中自发选择性肽延伸
大自然选择磷酸盐来激活氨基酸,其中反应性中间体和复杂的机械驱动聚酰胺的构建。在生物学之外,在水性非生物系统中允许自发和选择性肽延伸的途径和机制仍不清楚。在此,我们致力于通过跟踪氨酰磷酸酯(氨酰腺苷酸的合成对应物)的系统化学来揭示这些途径。磷酸酯充当溶解度标签,使疏水性氨基酸及其低聚物可溶于水,并实现选择性延伸和不同途径的出现。因此,在一个烧瓶中以分钟的时间尺度合成了直至十二聚体的低聚物,其中连续添加激活了自主相变。根据途径,所得相最初携带非极性肽和含有磷酸酯的两亲性低聚物。在伸长和磷酸盐释放过程中,较短的低聚物在溶液中占主导地位,而聚集相由于其自组装倾向而有利于较长低聚物的存在。此外,我们证明,通过添加各种磷酸酯,可以分离溶液相并充当连续伸长的新环境。这些发现表明,氨酰基磷酸酯的系统化学可以通过合并水相合成和自组装来激活肽键形成的选择机制。
更新日期:2023-11-22
中文翻译:

氨酰磷酸酯和相变驱动的水中自发选择性肽延伸
大自然选择磷酸盐来激活氨基酸,其中反应性中间体和复杂的机械驱动聚酰胺的构建。在生物学之外,在水性非生物系统中允许自发和选择性肽延伸的途径和机制仍不清楚。在此,我们致力于通过跟踪氨酰磷酸酯(氨酰腺苷酸的合成对应物)的系统化学来揭示这些途径。磷酸酯充当溶解度标签,使疏水性氨基酸及其低聚物可溶于水,并实现选择性延伸和不同途径的出现。因此,在一个烧瓶中以分钟的时间尺度合成了直至十二聚体的低聚物,其中连续添加激活了自主相变。根据途径,所得相最初携带非极性肽和含有磷酸酯的两亲性低聚物。在伸长和磷酸盐释放过程中,较短的低聚物在溶液中占主导地位,而聚集相由于其自组装倾向而有利于较长低聚物的存在。此外,我们证明,通过添加各种磷酸酯,可以分离溶液相并充当连续伸长的新环境。这些发现表明,氨酰基磷酸酯的系统化学可以通过合并水相合成和自组装来激活肽键形成的选择机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号