当前位置:
X-MOL 学术
›
Mol. Ther. Methods Clin. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CRISPR-Cas9-mediated somatic correction of a one-base deletion in the Ugt1a gene ameliorates hyperbilirubinemia in Crigler-Najjar syndrome mice
Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2023-11-19 , DOI: 10.1016/j.omtm.2023.101161 Giulia Bortolussi 1 , Alessandra Iaconcig 1 , Giulia Canarutto 1 , Fabiola Porro 1 , Filippo Ferrucci 1 , Claudia Galletta 1 , Cristian Díaz-Muñoz 1 , Vipin Rawat 1 , Alessia De Caneva 1 , Olayemi Joseph Olajide 1 , Lorena Zentilin 1 , Silvano Piazza 1 , Luka Bočkor 1 , Andrés Fernando Muro 1
Molecular Therapy - Methods & Clinical Development ( IF 4.6 ) Pub Date : 2023-11-19 , DOI: 10.1016/j.omtm.2023.101161 Giulia Bortolussi 1 , Alessandra Iaconcig 1 , Giulia Canarutto 1 , Fabiola Porro 1 , Filippo Ferrucci 1 , Claudia Galletta 1 , Cristian Díaz-Muñoz 1 , Vipin Rawat 1 , Alessia De Caneva 1 , Olayemi Joseph Olajide 1 , Lorena Zentilin 1 , Silvano Piazza 1 , Luka Bočkor 1 , Andrés Fernando Muro 1
Affiliation
|
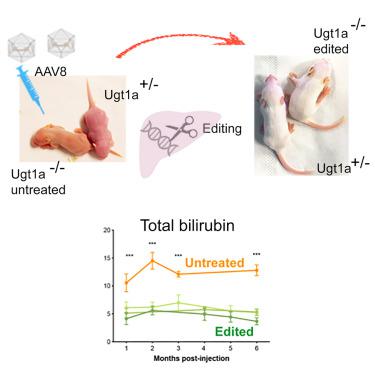
(AAV)-mediated episomal gene replacement therapy for monogenic liver disorders is currently limited in pediatric settings due to the loss of vector DNA, associated with hepatocyte duplication during liver growth. Genome editing is a promising strategy leading to a permanent and specific genome modification that is transmitted to daughter cells upon proliferation. Using genome targeting, we previously rescued neonatal lethality in mice with Crigler-Najjar syndrome. This rare monogenic disease is characterized by severe neonatal unconjugated hyperbilirubinemia, neurological damage, and death. Here, using the CRISPR- Cas9 (Cas9) platform, we edited the disease-causing mutation present in the Ugt1a locus of these mice. Newborn mice were treated with two AAV8 vectors: one expressing the Cas9 and single guide RNA, and the other carrying the homology regions with the corrected sequence, while maintained in a temporary phototherapy setting rescuing mortality. We observed a 50% plasma bilirubin reduction that remained stable for up to 6 months. We then tested different Cas9:donor vector ratios, with a 1:5 ratio showing the greatest efficacy in lowering plasma bilirubin, with partial lethality rescue when more severe, lethal conditions were applied. In conclusion, we reduced plasma bilirubin to safe levels and partially rescued neonatal lethality by correcting the mutant gene of a Crigler-Najjar mouse model.
中文翻译:

CRISPR-Cas9介导的Ugt1a基因一碱基缺失的体细胞校正可改善克里格勒-纳贾尔综合征小鼠的高胆红素血症
(AAV) 介导的附加型基因替代疗法治疗单基因肝病目前在儿科环境中受到限制,因为载体 DNA 的丢失与肝脏生长过程中肝细胞的复制有关。基因组编辑是一种有前景的策略,可导致永久性和特定的基因组修饰,并在增殖时传递给子细胞。通过基因组靶向,我们之前挽救了患有克里格勒-纳贾尔综合征的小鼠的新生儿死亡。这种罕见的单基因疾病的特点是严重的新生儿非结合胆红素血症、神经损伤和死亡。在这里,我们使用 CRISPR-Cas9 (Cas9) 平台编辑了这些小鼠 Ugt1a 基因座中存在的致病突变。新生小鼠接受两种 AAV8 载体治疗:一种表达 Cas9 和单引导 RNA,另一种携带具有正确序列的同源区域,同时维持在临时光疗环境中以挽救死亡率。我们观察到血浆胆红素降低了 50%,并且在长达 6 个月内保持稳定。然后,我们测试了不同的 Cas9: 供体载体比例,其中 1:5 的比例显示出降低血浆胆红素的最大功效,当应用更严重的致命条件时,可实现部分致死性挽救。总之,我们通过纠正 Crigler-Najjar 小鼠模型的突变基因,将血浆胆红素降低至安全水平,并部分挽救了新生儿死亡率。
更新日期:2023-11-19
中文翻译:

CRISPR-Cas9介导的Ugt1a基因一碱基缺失的体细胞校正可改善克里格勒-纳贾尔综合征小鼠的高胆红素血症
(AAV) 介导的附加型基因替代疗法治疗单基因肝病目前在儿科环境中受到限制,因为载体 DNA 的丢失与肝脏生长过程中肝细胞的复制有关。基因组编辑是一种有前景的策略,可导致永久性和特定的基因组修饰,并在增殖时传递给子细胞。通过基因组靶向,我们之前挽救了患有克里格勒-纳贾尔综合征的小鼠的新生儿死亡。这种罕见的单基因疾病的特点是严重的新生儿非结合胆红素血症、神经损伤和死亡。在这里,我们使用 CRISPR-Cas9 (Cas9) 平台编辑了这些小鼠 Ugt1a 基因座中存在的致病突变。新生小鼠接受两种 AAV8 载体治疗:一种表达 Cas9 和单引导 RNA,另一种携带具有正确序列的同源区域,同时维持在临时光疗环境中以挽救死亡率。我们观察到血浆胆红素降低了 50%,并且在长达 6 个月内保持稳定。然后,我们测试了不同的 Cas9: 供体载体比例,其中 1:5 的比例显示出降低血浆胆红素的最大功效,当应用更严重的致命条件时,可实现部分致死性挽救。总之,我们通过纠正 Crigler-Najjar 小鼠模型的突变基因,将血浆胆红素降低至安全水平,并部分挽救了新生儿死亡率。






























 京公网安备 11010802027423号
京公网安备 11010802027423号