European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-11-07 , DOI: 10.1016/j.ejmech.2023.115924 Su Yu 1 , Yan Zhang 2 , Jie Yang 1 , Hongrui Xu 3 , Suke Lan 4 , Binyan Zhao 1 , Meng Luo 1 , Xinyu Ma 1 , Hongjia Zhang 1 , Shirui Wang 1 , Hui Shen 3 , Yan Zhang 3 , Yong Xu 3 , Rui Li 1
|
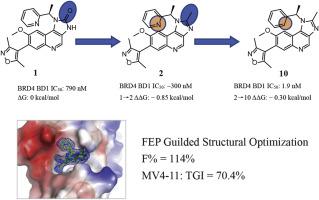
The functions of the bromodomain and extra terminal (BET) family of proteins have been proved to be involved in various diseases, particularly the acute myeloid leukemia (AML). In this work, guided by free energy perturbation (FEP) calculation, a methyl group was selected to be attached to the 1H-imidazo[4,5-c]quinoline skeleton, and a series of congeneric compounds were synthesized. Among them, compound 10 demonstrated outstanding activity against BRD4 BD1 with an IC50 value of 1.9 nM and exhibited remarkable antiproliferative effects against MV4-11 cells. The X-ray cocrystal structure proved that 10 occupied the acetylated lysine (KAc) binding cavity and the WPF shelf of BRD4 BD1. Additionally, 10 displayed high selectivity towards BET family members, effectively inhibiting the growth of AML cells, promoting apoptosis, and arresting the cell cycle at the G0/G1 phase. Further mechanistic studies demonstrated that compound 10 could suppress the expression of c-Myc and CDK6 while enhancing the expression of P21, PARP, and cleaved PARP. Moreover, 10 exhibited remarkable pharmacokinetic properties and significant antitumor efficacy in vivo. Therefore, compound 10 may represent a new, potent and selective BET bromodomain inhibitor for the development of therapeutics to treat AML.
中文翻译:

发现(R)-4-(8-甲氧基-2-甲基-1-(1-苯基乙基)-1H-咪唑并[4,5-c]喹啉-7-基)-3,5-二甲基异恶唑作为有效的和基于 FEP 计算指导的选择性 BET 抑制剂治疗急性髓系白血病 (AML)
溴结构域和额外末端(BET)蛋白家族的功能已被证明与多种疾病有关,特别是急性髓系白血病(AML)。本工作以自由能微扰(FEP)计算为指导,选择甲基连接到1H-咪唑并[4,5- c ]喹啉骨架上,合成了一系列同系化合物。其中,化合物10对BRD4 BD1表现出优异的活性,IC 50值为1.9 nM,并对MV4-11细胞表现出显着的抗增殖作用。 X射线共晶结构证明10占据了BRD4 BD1的乙酰化赖氨酸(KAc)结合腔和WPF架。此外, 10对BET家族成员表现出高选择性,有效抑制AML细胞的生长,促进细胞凋亡,并将细胞周期阻滞在G 0 /G 1期。进一步的机制研究表明,化合物10可以抑制c-Myc和CDK6的表达,同时增强P21 、PARP和裂解的PARP的表达。此外, 10在体内表现出显着的药代动力学特性和显着的抗肿瘤功效。因此,化合物10可能代表一种新的、有效的、选择性的BET溴结构域抑制剂,用于开发治疗AML的疗法。






























 京公网安备 11010802027423号
京公网安备 11010802027423号