Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-11-14 , DOI: 10.1016/j.molliq.2023.123583
Lu Zheng , Qiuke Li , Siqi Fang , Xinyue Zhang , Hongwei Zhang , Zhenping Cai , Kuan Huang , Lilong Jiang
|
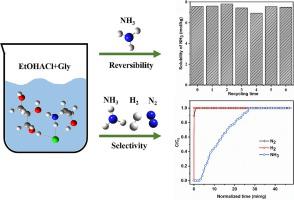
Efficiently separating NH3 from the exit gas of NH3 synthesis tower has significant economic and environmental benefits, such as improving the NH3 conversion rate and reducing pollution emission. In this study, a series of EtOHACl + Gly deep eutectic solvents (DESs) with numerous hydrogen-bond sites were synthesized. The findings suggest that the use of EtOHACl + Gly DESs can result in both exceptional NH3 absorption capacities and superior selectivities. EtOHACl + Gly (1:2) has the NH3 capacities of up to 12.39 mol/kg at 298.2 K and 105.5 kPa, while its N2 and H2 capacities are negligible. Additionally, the absorbed NH3 can be easily released by heating and reducing pressure, and the NH3 capacities remain stable after undergoing six cycles. The study also examined the interaction mechanism of EtOHACl + Gly DESs for the NH3 separation, utilizing spectral characterizations, quantum chemistry calculations and molecular dynamics simulations.
中文翻译:

氯化乙醇铵-甘油低共熔溶剂通过多重氢键相互作用有效可逆吸收 NH3
从NH 3合成塔出口气体中高效分离NH 3,具有提高NH 3转化率、减少污染排放等显着的经济效益和环境效益。在这项研究中,合成了一系列具有大量氢键位点的 EtOHACl + Gly 低共熔溶剂 (DES)。研究结果表明,使用 EtOHACl + Gly DES 可以实现卓越的 NH 3吸收能力和优异的选择性。EtOHACl + Gly (1:2) 在298.2 K和105.5 kPa下的NH 3容量高达12.39 mol/kg,而其N 2和H 2容量可以忽略不计。此外,所吸收的NH 3可以通过加热和减压轻松释放,并且NH 3容量在经历六个循环后仍保持稳定。该研究还利用光谱表征、量子化学计算和分子动力学模拟,研究了 EtOHACl + Gly DES 用于 NH 3分离的相互作用机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号