当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Operando Mechanistic Studies of CO2 Hydrogenation by Ruthenium Complexes Using High-Pressure NMR Spectroscopy
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-11-20 , DOI: 10.1021/acscatal.3c03908
Brandon R. Galan 1 , Jennifer O. Bigelow 1 , William G. Dougherty 2 , W. Scott Kassel 2 , Elliott B. Hulley 1 , Monte L. Helm 1 , M. Rakowski DuBois 1 , Aaron M. Appel 1 , John C. Linehan 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-11-20 , DOI: 10.1021/acscatal.3c03908
Brandon R. Galan 1 , Jennifer O. Bigelow 1 , William G. Dougherty 2 , W. Scott Kassel 2 , Elliott B. Hulley 1 , Monte L. Helm 1 , M. Rakowski DuBois 1 , Aaron M. Appel 1 , John C. Linehan 1
Affiliation
|
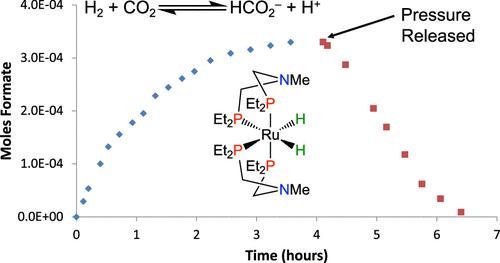
As a result of increased energy demands, the ability to both reduce carbon dioxide with dihydrogen to formate and conduct the reverse reaction with the same catalyst is of interest as a method for potential fuel generation and use. Ruthenium bis(diphosphine) complexes with and without pendant amines were reacted with mixtures of CO2/H2 gases in the presence of added base to catalytically yield formate; when the base was triethylamine, the reaction was found to be reversible. The reactions were monitored using high-pressure operando 1H and 31P{1H} NMR spectroscopy at 18 °C in THF under 40 atm of a 1:1 mixture of H2 and CO2. The rate of production of formate was correlated with the observation of specific organometallic species by NMR spectroscopy under catalytic conditions, including a hydrido–dihydrogen complex. From this operando study, a mechanism is proposed with two competing catalytic cycles for which the predominant cycle is dependent on which base and catalyst are used. The role of the base is shown to be vital for both catalytic rate and reversibility of the chemical transformation, indicating base selection should be carefully considered.
中文翻译:

使用高压核磁共振波谱法研究钌配合物加氢 CO2 的操作机理
由于能源需求的增加,用氢气还原二氧化碳以形成并用相同的催化剂进行逆反应的能力作为潜在的燃料生成和使用的方法是令人感兴趣的。具有和不具有侧胺的钌双(二膦)配合物与CO 2 /H 2气体混合物在添加的碱存在下反应以催化生成甲酸盐;当碱为三乙胺时,发现该反应是可逆的。使用高压操作 1 H和31 P{ 1 H} NMR光谱在18℃下在THF中在40atm的H 2和CO 2的1:1混合物中监测反应。甲酸盐的生成速率与在催化条件下通过核磁共振波谱观察到的特定有机金属物质(包括氢化二氢络合物)相关。根据这项操作研究,提出了一种具有两个竞争催化循环的机制,其中主要循环取决于所使用的碱和催化剂。碱的作用对于化学转化的催化速率和可逆性都至关重要,这表明碱的选择应该仔细考虑。
更新日期:2023-11-20
中文翻译:

使用高压核磁共振波谱法研究钌配合物加氢 CO2 的操作机理
由于能源需求的增加,用氢气还原二氧化碳以形成并用相同的催化剂进行逆反应的能力作为潜在的燃料生成和使用的方法是令人感兴趣的。具有和不具有侧胺的钌双(二膦)配合物与CO 2 /H 2气体混合物在添加的碱存在下反应以催化生成甲酸盐;当碱为三乙胺时,发现该反应是可逆的。使用高压操作 1 H和31 P{ 1 H} NMR光谱在18℃下在THF中在40atm的H 2和CO 2的1:1混合物中监测反应。甲酸盐的生成速率与在催化条件下通过核磁共振波谱观察到的特定有机金属物质(包括氢化二氢络合物)相关。根据这项操作研究,提出了一种具有两个竞争催化循环的机制,其中主要循环取决于所使用的碱和催化剂。碱的作用对于化学转化的催化速率和可逆性都至关重要,这表明碱的选择应该仔细考虑。

































 京公网安备 11010802027423号
京公网安备 11010802027423号