当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Naphthyl bearing 1,3,4-thiadiazoleacetamides targeting the parasitic folate pathway as anti-infectious agents: in silico, synthesis, and biological approach
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2023-11-21 , DOI: 10.1039/d3md00423f Kavita Pal 1 , Sahil Lala 2, 3 , Priyanka Agarwal 2, 3 , Tarosh S Patel 4 , Jenny Legac 5 , Md Ataur Rahman 6 , Saiema Ahmedi 7 , Nida Shahid 8 , Sneha Singh 9 , Kajal Kumari 9 , Hari Madhav 1 , Abhik Sen 9 , Nikhat Manzoor 7 , Bharat C Dixit 4 , Robyn Van Zyl 2, 3 , Philip J Rosenthal 5 , Nasimul Hoda 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2023-11-21 , DOI: 10.1039/d3md00423f Kavita Pal 1 , Sahil Lala 2, 3 , Priyanka Agarwal 2, 3 , Tarosh S Patel 4 , Jenny Legac 5 , Md Ataur Rahman 6 , Saiema Ahmedi 7 , Nida Shahid 8 , Sneha Singh 9 , Kajal Kumari 9 , Hari Madhav 1 , Abhik Sen 9 , Nikhat Manzoor 7 , Bharat C Dixit 4 , Robyn Van Zyl 2, 3 , Philip J Rosenthal 5 , Nasimul Hoda 1
Affiliation
|
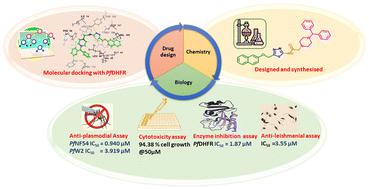
Malaria is still a complex and lethal parasitic infectious disease, despite the availability of effective antimalarial drugs. Resistance of malaria parasites to current treatments necessitates new antimalarials targeting P. falciparum proteins. The present study reported the design and synthesis of a series of a 2-(4-substituted piperazin-1-yl)-N-(5-((naphthalen-2-yloxy)methyl)-1,3,4-thiadiazol-2-yl)acetamide hybrids for the inhibition of Plasmodium falciparum dihydrofolate reductase (PfDHFR) using computational biology tools followed by chemical synthesis, structural characterization, and functional analysis. The synthesized compounds were evaluated for their in vitro antimalarial activity against CQ-sensitive PfNF54 and CQ-resistant PfW2 strain. Compounds T5 and T6 are the most active compounds having anti-plasmodial activity against PfNF54 with IC50 values of 0.94 and 3.46 μM respectively. Compound T8 is the most active against the PfW2 strain having an IC50 of 3.91 μM. Further, these active hybrids (T5, T6, and T8) were also evaluated for enzyme inhibition assay against PfDHFR. All the tested compounds were non-toxic against the Hek293 cell line with good selectivity indices. Hemolysis assay also showed non-toxicity of these compounds on normal uninfected human RBCs. In silico molecular docking studies were carried out in the binding pocket of both the wild-type and quadruple mutant Pf-DHFR-TS to gain further insights into probable modes of action of active compounds. ADME prediction and physiochemical properties support their drug-likeness. Additionally, they were screened for antileishmanial activity against L. donovani promastigotes to explore broader applications. Thus, this study provides molecular frameworks for developing potent antimalarials and antileishmanial agents.
中文翻译:

靶向寄生叶酸途径的携带 1,3,4-噻二唑乙酰胺作为抗感染剂:计算机模拟、合成和生物学方法
尽管有有效的抗疟药物,但疟疾仍然是一种复杂且致命的寄生虫传染病。疟疾寄生虫对当前治疗的耐药性需要针对恶性疟原虫蛋白的新型抗疟药。本研究报道了一系列 2-(4-取代哌嗪-1-基)-N-(5-(萘-2-基氧基)甲基)-1,3,4-噻二唑-2-基)乙酰胺杂交体的设计和合成,用于抑制恶性疟原虫二氢叶酸还原酶 (PfDHFR),随后使用计算生物学工具进行化学合成、结构表征和功能分析。评价了合成化合物对 CQ 敏感的 PfNF54 和 CQ 耐药的 PfW2 菌株的体外抗疟活性。化合物 T5 和 T6 是对 PfNF54 具有抗疟原虫活性的最活跃化合物,IC50 值分别为 0.94 和 3.46 μM。化合物 T8 对 PfW2 菌株最活跃,IC50 为 3.91 μM。此外,还评估了这些活性杂交体 (T5 、 T6 和 T8) 用于针对 PfDHFR 的酶抑制测定。所有供试化合物对 Hek293 细胞系均无毒,具有良好的选择性指标。溶血试验还显示这些化合物对正常未感染的人红细胞无毒。在野生型和四重突变体 Pf-DHFR-TS 的结合口袋中进行了计算机分子对接研究,以进一步了解活性化合物的可能作用模式。 ADME 预测和理化性质支持其药物相似性。此外,还筛选了它们对 L. donovani 前鞭毛体的抗利什曼原虫活性,以探索更广泛的应用。因此,本研究为开发有效的抗疟药和抗利什曼原虫药物提供了分子框架。
更新日期:2023-11-21
中文翻译:

靶向寄生叶酸途径的携带 1,3,4-噻二唑乙酰胺作为抗感染剂:计算机模拟、合成和生物学方法
尽管有有效的抗疟药物,但疟疾仍然是一种复杂且致命的寄生虫传染病。疟疾寄生虫对当前治疗的耐药性需要针对恶性疟原虫蛋白的新型抗疟药。本研究报道了一系列 2-(4-取代哌嗪-1-基)-N-(5-(萘-2-基氧基)甲基)-1,3,4-噻二唑-2-基)乙酰胺杂交体的设计和合成,用于抑制恶性疟原虫二氢叶酸还原酶 (PfDHFR),随后使用计算生物学工具进行化学合成、结构表征和功能分析。评价了合成化合物对 CQ 敏感的 PfNF54 和 CQ 耐药的 PfW2 菌株的体外抗疟活性。化合物 T5 和 T6 是对 PfNF54 具有抗疟原虫活性的最活跃化合物,IC50 值分别为 0.94 和 3.46 μM。化合物 T8 对 PfW2 菌株最活跃,IC50 为 3.91 μM。此外,还评估了这些活性杂交体 (T5 、 T6 和 T8) 用于针对 PfDHFR 的酶抑制测定。所有供试化合物对 Hek293 细胞系均无毒,具有良好的选择性指标。溶血试验还显示这些化合物对正常未感染的人红细胞无毒。在野生型和四重突变体 Pf-DHFR-TS 的结合口袋中进行了计算机分子对接研究,以进一步了解活性化合物的可能作用模式。 ADME 预测和理化性质支持其药物相似性。此外,还筛选了它们对 L. donovani 前鞭毛体的抗利什曼原虫活性,以探索更广泛的应用。因此,本研究为开发有效的抗疟药和抗利什曼原虫药物提供了分子框架。






























 京公网安备 11010802027423号
京公网安备 11010802027423号