当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessing the Performance Limits of Electrochemical CO2 Separation Using Exergy Loss Analysis and Zero-Dimensional Modeling
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-11-21 , DOI: 10.1021/acssuschemeng.3c04867 Fawaz Ali 1 , Sanat Modak 1 , David G. Kwabi 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-11-21 , DOI: 10.1021/acssuschemeng.3c04867 Fawaz Ali 1 , Sanat Modak 1 , David G. Kwabi 1
Affiliation
|
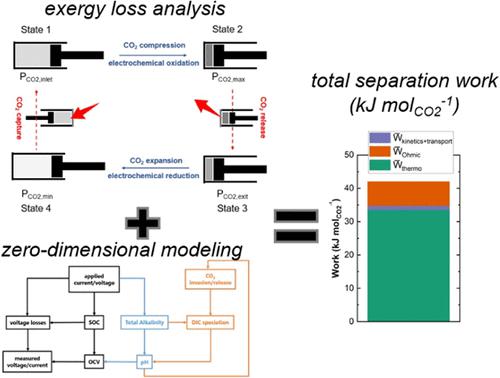
Electrochemical CO2 separation is drawing attention as a promising strategy for using renewable energy to mitigate climate change. Several studies have shown that the minimum energy input thermodynamically required for such separation, either via pH-swing or nucleophilic binding of CO2 to a charge carrier, can be very low (<100 kJ molCO2–1). Nevertheless, understanding how measured energetic costs are likely to vary as a function of various electrochemical and separation process parameters in practical applications is not fully understood. In this study, we first show that the minimum energy required to execute any electrochemical CO2 separation cycle is a function of the exergy losses incurred by the entry of CO2 into the electrolyte (from a dilute inlet feed) or the release of CO2 from the electrolyte (into a more concentrated exit stream). These exergy losses can be calculated based on the trajectories of solution- and gas-phase concentrations of CO2 throughout a given separation cycle; they do not depend on the separation mechanism (i.e., whether related to pH-swing or direct binding of CO2 to a nucleophilic charge carrier). Then, we develop a zero-dimensional model of pH-swing-driven CO2 separation in a flow cell, which incorporates the physics of reactive CO2 capture into alkaline media with the cell’s electrochemical operation. From the output of the model, we show how the overall energetic cost of separation can be decomposed into distinct contributions arising from exergy and other flow cell-specific losses (i.e., to interfacial charge transfer, mass transport, and Ohmic resistance of the cell). Variations in energetic cost as a function of CO2 removed, current density, and the rate constant of CO2 hydroxylation are explored. Our investigations reveal that when CO2 capture is rate-limiting for the overall separation process, the trade-off/relationship between energy input and the CO2 throughput is strongly dictated by the difference between the time allotted for CO2 invasion during cycling and the time required for reactive capture of CO2. When those timescales are comparable, the energy input and CO2 throughput are optimally low and high, respectively. Our work contributes toward ongoing efforts to design electrochemical systems that could meet the techno-economic requirements for practically viable CO2 separation.
中文翻译:

使用火用损失分析和零维建模评估电化学 CO2 分离的性能极限
电化学CO 2分离作为利用可再生能源减缓气候变化的一种有前景的策略而受到关注。多项研究表明,这种分离所需的热力学最小能量输入(通过 pH 波动或 CO 2与电荷载体的亲核结合)可能非常低(<100 kJ mol CO 2 –1)。然而,在实际应用中,测量的能量成本可能如何随着各种电化学和分离过程参数的变化而变化,这一点尚不完全清楚。在本研究中,我们首先表明,执行任何电化学 CO 2分离循环所需的最小能量是 CO 2进入电解质(来自稀释入口进料)或释放 CO 2所引起的火用损失的函数从电解质中(进入更浓缩的出口流)。这些火用损失可以根据整个给定分离周期中CO 2溶液和气相浓度的轨迹来计算;它们不依赖于分离机制(即是否与pH波动有关或CO 2与亲核电荷载体的直接结合有关)。然后,我们开发了流通池中pH 波动驱动的 CO 2分离的零维模型,该模型将反应性 CO 2捕获到碱性介质中的物理原理与电池的电化学操作相结合。从模型的输出中,我们展示了如何将分离的总体能量成本分解为由㶲和其他流动池特定损失(即界面电荷转移、质量传输和电池的欧姆电阻)引起的不同贡献。探索了能量成本随去除的CO 2 、电流密度和CO 2羟基化速率常数的变化。我们的研究表明,当 CO 2捕获是整个分离过程的速率限制时,能量输入和 CO 2吞吐量之间的权衡/关系很大程度上取决于循环期间分配给 CO 2侵入的时间与反应捕获CO 2所需的时间。当这些时间尺度具有可比性时,能量输入和CO 2吞吐量分别最佳地低和高。我们的工作有助于不断努力设计电化学系统,以满足实际可行的CO 2分离的技术经济要求。
更新日期:2023-11-21
中文翻译:

使用火用损失分析和零维建模评估电化学 CO2 分离的性能极限
电化学CO 2分离作为利用可再生能源减缓气候变化的一种有前景的策略而受到关注。多项研究表明,这种分离所需的热力学最小能量输入(通过 pH 波动或 CO 2与电荷载体的亲核结合)可能非常低(<100 kJ mol CO 2 –1)。然而,在实际应用中,测量的能量成本可能如何随着各种电化学和分离过程参数的变化而变化,这一点尚不完全清楚。在本研究中,我们首先表明,执行任何电化学 CO 2分离循环所需的最小能量是 CO 2进入电解质(来自稀释入口进料)或释放 CO 2所引起的火用损失的函数从电解质中(进入更浓缩的出口流)。这些火用损失可以根据整个给定分离周期中CO 2溶液和气相浓度的轨迹来计算;它们不依赖于分离机制(即是否与pH波动有关或CO 2与亲核电荷载体的直接结合有关)。然后,我们开发了流通池中pH 波动驱动的 CO 2分离的零维模型,该模型将反应性 CO 2捕获到碱性介质中的物理原理与电池的电化学操作相结合。从模型的输出中,我们展示了如何将分离的总体能量成本分解为由㶲和其他流动池特定损失(即界面电荷转移、质量传输和电池的欧姆电阻)引起的不同贡献。探索了能量成本随去除的CO 2 、电流密度和CO 2羟基化速率常数的变化。我们的研究表明,当 CO 2捕获是整个分离过程的速率限制时,能量输入和 CO 2吞吐量之间的权衡/关系很大程度上取决于循环期间分配给 CO 2侵入的时间与反应捕获CO 2所需的时间。当这些时间尺度具有可比性时,能量输入和CO 2吞吐量分别最佳地低和高。我们的工作有助于不断努力设计电化学系统,以满足实际可行的CO 2分离的技术经济要求。































 京公网安备 11010802027423号
京公网安备 11010802027423号