当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The state of Fe3+ in the C–F–A–S–H system with varying Fe/Si and Ca/Si ratios
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-11-22 , DOI: 10.1039/d3ta01929b
Yuan Fang 1 , Kunde Zhuang 1 , Hongzhi Cui 1 , Zuhua Zhang 2, 3 , Aoxuan Wang 1 , Chenman Wang 1 , Dapeng Zheng 1 , Xianfeng Wang 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-11-22 , DOI: 10.1039/d3ta01929b
Yuan Fang 1 , Kunde Zhuang 1 , Hongzhi Cui 1 , Zuhua Zhang 2, 3 , Aoxuan Wang 1 , Chenman Wang 1 , Dapeng Zheng 1 , Xianfeng Wang 1
Affiliation
|
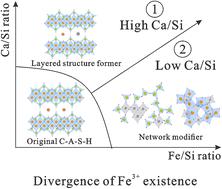
Synthetic CaO–Fe2O3–Al2O3–SiO2–H2O (C–F–A–S–H) gels with Fe/Si and Ca/Si ratios in the ranges 1/8–1/4 and 1.0–2.0, respectively, are investigated to reveal the coordination, location, and doping configuration of Fe3+. The bonding between Fe3+ and Ca2+/Si4+ species is the most favorable, resulting in the transformation from phyllosilicates (i.e., layered C–A–S–H) to network silicates (hydroandradite and disordered gel with a crosslinked structure). The increasing Ca/Si ratio has a critical effect on the evolution of the C–F–A–S–H system, resulting in layered C–(F)–A–S–H because a sufficient concentration of Ca ions is provided. Using Mössbauer spectroscopy, two kinds of octahedral Fe3+ with distinct chemical environments are identified: the distorted one acts as a network modifier, while the highly symmetric one acts as a layered structure former. The former exists in the disordered gel, while the latter mainly exists in the interlayer space of the layered C–(F)–A–S–H. Moreover, Fe3+ is unlikely to enter the tetrahedral position of the aluminosilicate chain in layered C–(F)–A–S–H.
中文翻译:

具有不同 Fe/Si 和 Ca/Si 比率的 C-F-A-S-H 体系中 Fe3+ 的状态
合成 CaO–Fe 2 O 3 –Al 2 O 3 –SiO 2 –H 2 O (C–F–A–S–H) 凝胶,Fe/Si 和 Ca/Si 比率在 1/8–1/4 范围内分别研究了Fe 3+ 和1.0–2.0,以揭示Fe 3+ 的配位、位置和掺杂构型。Fe 3+和 Ca 2+ /Si 4+物质之间的键合是最有利的,导致从页硅酸盐(即层状 C–A–S–H)转变为网络硅酸盐(水钙铁矿和具有交联结构的无序凝胶) )。Ca/Si 比率的增加对 C-F-A-S-H 体系的演化具有关键影响,由于提供了足够浓度的 Ca 离子,导致层状 C-(F)-A-S-H。利用穆斯堡尔谱,鉴定出两种具有不同化学环境的八面体 Fe 3+ :扭曲的 Fe 3+ 充当网络改性剂,而高度对称的 Fe 3+ 充当层状结构形成者。前者存在于无序凝胶中,后者主要存在于层状C–(F)–A–S–H的层间空间中。此外,Fe 3+不太可能进入层状C–(F)–A–S–H 中铝硅酸盐链的四面体位置。
更新日期:2023-11-22
中文翻译:

具有不同 Fe/Si 和 Ca/Si 比率的 C-F-A-S-H 体系中 Fe3+ 的状态
合成 CaO–Fe 2 O 3 –Al 2 O 3 –SiO 2 –H 2 O (C–F–A–S–H) 凝胶,Fe/Si 和 Ca/Si 比率在 1/8–1/4 范围内分别研究了Fe 3+ 和1.0–2.0,以揭示Fe 3+ 的配位、位置和掺杂构型。Fe 3+和 Ca 2+ /Si 4+物质之间的键合是最有利的,导致从页硅酸盐(即层状 C–A–S–H)转变为网络硅酸盐(水钙铁矿和具有交联结构的无序凝胶) )。Ca/Si 比率的增加对 C-F-A-S-H 体系的演化具有关键影响,由于提供了足够浓度的 Ca 离子,导致层状 C-(F)-A-S-H。利用穆斯堡尔谱,鉴定出两种具有不同化学环境的八面体 Fe 3+ :扭曲的 Fe 3+ 充当网络改性剂,而高度对称的 Fe 3+ 充当层状结构形成者。前者存在于无序凝胶中,后者主要存在于层状C–(F)–A–S–H的层间空间中。此外,Fe 3+不太可能进入层状C–(F)–A–S–H 中铝硅酸盐链的四面体位置。

































 京公网安备 11010802027423号
京公网安备 11010802027423号