当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Imidazolone as an Amide Bioisostere in the Development of β-1,3-N-Acetylglucosaminyltransferase 2 (B3GNT2) Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-11-21 , DOI: 10.1021/acs.jmedchem.3c01517
Jeffrey J Jackson 1 , Aaron C Siegmund 1 , Wen-Ju Bai 1 , Anthony B Reed 1 , Adam B Birkholz 2 , Iain D G Campuzano 2 , Amandine Créquer-Grandhomme 3 , Ruozhen Hu 3 , Rucha V Modak 3 , Athena Sudom 1 , Noelle Javier 4 , Christiana Sanders 5 , Mei-Chu Lo 4 , Fang Xie 6 , Victor J Cee 1 , Paolo Manzanillo 3 , John G Allen 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-11-21 , DOI: 10.1021/acs.jmedchem.3c01517
Jeffrey J Jackson 1 , Aaron C Siegmund 1 , Wen-Ju Bai 1 , Anthony B Reed 1 , Adam B Birkholz 2 , Iain D G Campuzano 2 , Amandine Créquer-Grandhomme 3 , Ruozhen Hu 3 , Rucha V Modak 3 , Athena Sudom 1 , Noelle Javier 4 , Christiana Sanders 5 , Mei-Chu Lo 4 , Fang Xie 6 , Victor J Cee 1 , Paolo Manzanillo 3 , John G Allen 1
Affiliation
|
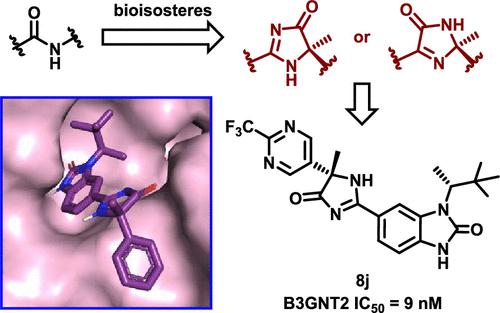
B3GNT2 is responsible for elongation of cell surface long-chain polylactosamine, which influences the regulation of the immune response, making it an attractive target for immunomodulation. In the development of amide containing B3GNT2 inhibitors guided by structure-based drug design, imidazolones were found to successfully serve as amide bioisosteres. This novel imidazolone isosteric strategy alleviated torsional strain of the amide bond on binding to B3GNT2 and improved potency, isoform selectivity, as well as certain physicochemical and pharmacokinetic properties. Herein, we present the synthesis, SAR, X-ray cocrystal structures, and in vivo PK properties of imidazol-4-ones in the context of B3GNT2 inhibition.
中文翻译:

咪唑酮作为酰胺生物等排体用于开发 β-1,3-N-乙酰葡糖胺基转移酶 2 (B3GNT2) 抑制剂
B3GNT2 负责细胞表面长链聚乳糖胺的延长,从而影响免疫反应的调节,使其成为免疫调节的有吸引力的靶点。在以基于结构的药物设计为指导的含酰胺 B3GNT2 抑制剂的开发过程中,发现咪唑啉酮可以成功地充当酰胺生物等排体。这种新颖的咪唑酮等排策略减轻了与 B3GNT2 结合时酰胺键的扭转应变,并提高了效力、异构体选择性以及某些理化和药代动力学特性。在此,我们介绍了咪唑-4-酮在 B3GNT2 抑制中的合成、SAR、X 射线共晶结构和体内PK 特性。
更新日期:2023-11-21
中文翻译:

咪唑酮作为酰胺生物等排体用于开发 β-1,3-N-乙酰葡糖胺基转移酶 2 (B3GNT2) 抑制剂
B3GNT2 负责细胞表面长链聚乳糖胺的延长,从而影响免疫反应的调节,使其成为免疫调节的有吸引力的靶点。在以基于结构的药物设计为指导的含酰胺 B3GNT2 抑制剂的开发过程中,发现咪唑啉酮可以成功地充当酰胺生物等排体。这种新颖的咪唑酮等排策略减轻了与 B3GNT2 结合时酰胺键的扭转应变,并提高了效力、异构体选择性以及某些理化和药代动力学特性。在此,我们介绍了咪唑-4-酮在 B3GNT2 抑制中的合成、SAR、X 射线共晶结构和体内PK 特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号