当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Evaluation of C6-Substituted Pyrazolopyrimidine-Based Bisphosphonate Inhibitors of the Human Geranylgeranyl Pyrophosphate Synthase and Evaluation of Their Antitumor Efficacy in Multiple Myeloma, Pancreatic Ductal Adenocarcinoma, and Colorectal Cancer
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-11-20 , DOI: 10.1021/acs.jmedchem.3c01271 Rebecca Boutin 1 , Hiu-Fung Lee 1 , Tian Lai Guan 1, 2 , Tan Trieu Nguyen 3 , Xian Fang Huang 3 , Daniel D Waller 4 , Jordan Lu 5 , Iok In Christine Chio 5, 6 , René P Michel 7 , Michael Sebag 3, 8 , Youla S Tsantrizos 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-11-20 , DOI: 10.1021/acs.jmedchem.3c01271 Rebecca Boutin 1 , Hiu-Fung Lee 1 , Tian Lai Guan 1, 2 , Tan Trieu Nguyen 3 , Xian Fang Huang 3 , Daniel D Waller 4 , Jordan Lu 5 , Iok In Christine Chio 5, 6 , René P Michel 7 , Michael Sebag 3, 8 , Youla S Tsantrizos 1, 2
Affiliation
|
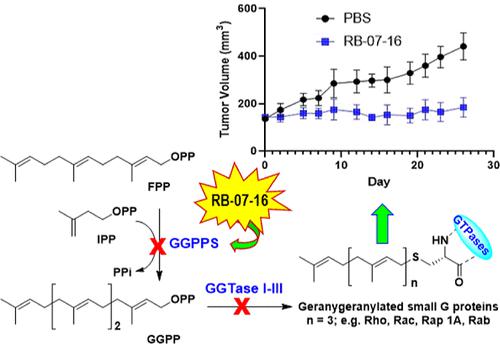
Novel C6-substituted pyrazolo[3,4-d]pyrimidine- and C2-substituted purine-based bisphosphonate (C6-PyraP-BP and C2-Pur-BP, respectively) inhibitors of the human geranylgeranyl pyrophosphate synthase (hGGPPS) were designed and evaluated for their ability to block the proliferation of multiple myeloma (MM), pancreatic ductal adenocarcinoma (PDAC), and colorectal cancer (CRC) cells. Pyrazolo[3,4-d]pyrimidine analogs were identified that induce selective intracellular target engagement leading to apoptosis and downregulate the prenylation of Rap-1A in MM, PDAC, and CRC cells. The C6-PyraP-BP inhibitor RB-07-16 was found to exhibit antitumor efficacy in xenograft mouse models of MM and PDAC, significantly reducing tumor growth without substantially increasing liver enzymes or causing significant histopathologic damage, usually associated with hepatotoxicity. RB-07-16 is a metabolically stable compound in cross-species liver microsomes, does not inhibit key CYP 450 enzymes, and exhibits good systemic circulation in rat. Collectively, the current studies provide encouraging support for further optimization of the pyrazolo[3,4-d]pyrimidine-based GGPPS inhibitors as potential human therapeutics for various cancers.
中文翻译:

人香叶基香叶基焦磷酸合酶 C6 取代的吡唑并嘧啶双膦酸盐抑制剂的发现和评估及其在多发性骨髓瘤、胰管腺癌和结直肠癌中的抗肿瘤功效
设计了新型 C6 取代的吡唑并[3,4- d ]嘧啶和 C2 取代的嘌呤基双膦酸盐(分别为 C6-PyraP-BP 和 C2-Pur-BP)人香叶基香叶基焦磷酸合酶 (hGGPPS) 抑制剂,并评估了它们阻止多发性骨髓瘤 (MM)、胰腺导管腺癌 (PDAC) 和结直肠癌 (CRC) 细胞增殖的能力。吡唑并[3,4- d ]嘧啶类似物经鉴定可诱导选择性细胞内靶标结合,导致细胞凋亡并下调 MM、PDAC 和 CRC 细胞中 Rap-1A 的异戊二烯化。 C6-PyraP-BP 抑制剂 RB-07-16 在 MM 和 PDAC 异种移植小鼠模型中表现出抗肿瘤功效,显着减少肿瘤生长,而不会显着增加肝酶或引起通常与肝毒性相关的显着组织病理学损伤。 RB-07-16 是跨物种肝微粒体中代谢稳定的化合物,不抑制关键的 CYP 450 酶,并且在大鼠中表现出良好的体循环。总的来说,当前的研究为进一步优化基于吡唑并[3,4- d ]嘧啶的GGPPS抑制剂作为多种癌症的潜在人类疗法提供了令人鼓舞的支持。
更新日期:2023-11-20
中文翻译:

人香叶基香叶基焦磷酸合酶 C6 取代的吡唑并嘧啶双膦酸盐抑制剂的发现和评估及其在多发性骨髓瘤、胰管腺癌和结直肠癌中的抗肿瘤功效
设计了新型 C6 取代的吡唑并[3,4- d ]嘧啶和 C2 取代的嘌呤基双膦酸盐(分别为 C6-PyraP-BP 和 C2-Pur-BP)人香叶基香叶基焦磷酸合酶 (hGGPPS) 抑制剂,并评估了它们阻止多发性骨髓瘤 (MM)、胰腺导管腺癌 (PDAC) 和结直肠癌 (CRC) 细胞增殖的能力。吡唑并[3,4- d ]嘧啶类似物经鉴定可诱导选择性细胞内靶标结合,导致细胞凋亡并下调 MM、PDAC 和 CRC 细胞中 Rap-1A 的异戊二烯化。 C6-PyraP-BP 抑制剂 RB-07-16 在 MM 和 PDAC 异种移植小鼠模型中表现出抗肿瘤功效,显着减少肿瘤生长,而不会显着增加肝酶或引起通常与肝毒性相关的显着组织病理学损伤。 RB-07-16 是跨物种肝微粒体中代谢稳定的化合物,不抑制关键的 CYP 450 酶,并且在大鼠中表现出良好的体循环。总的来说,当前的研究为进一步优化基于吡唑并[3,4- d ]嘧啶的GGPPS抑制剂作为多种癌症的潜在人类疗法提供了令人鼓舞的支持。































 京公网安备 11010802027423号
京公网安备 11010802027423号